Proteomics characterization of cell membrane blebs in human retinal pigment epithelium cells
- PMID: 19567368
- PMCID: PMC2758750
- DOI: 10.1074/mcp.M900203-MCP200
Proteomics characterization of cell membrane blebs in human retinal pigment epithelium cells
Abstract
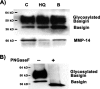
Age-related macular degeneration (AMD) is the leading cause of legal blindness among the elderly population in the industrialized world, affecting about 14 million people in the United States alone. Smoking is a major environmental risk factor for AMD, and hydroquinone is a major component in cigarette smoke. Hydroquinone induces the formation of cell membrane blebs in human retinal pigment epithelium (RPE). Blebs may accumulate and eventually contribute first to sub-RPE deposits and then drusen formation, which is a prominent histopathologic feature in eyes with AMD. As an attempt to better understand the mechanisms involved in early AMD, we sought to investigate the proteomic profile of RPE blebs. Isolated blebs were subjected to SDS-PAGE fractionation, and in-gel trypsin-digested peptides were analyzed by LC-MS/MS that lead to the identification of a total of 314 proteins. Identified proteins were predominantly involved in oxidative phosphorylation, cell junction, focal adhesion, cytoskeleton regulation, and immunogenic processes. Importantly basigin and matrix metalloproteinase-14, key proteins involved in extracellular matrix remodeling, were identified in RPE blebs and shown to be more prevalent in AMD patients. Altogether our findings suggest, for the first time, the potential involvement of RPE blebs in eye disease and shed light on the implication of cell-derived microvesicles in human pathology.
Figures





References
-
- Christen W. G., Glynn R. J., Manson J. E., Ajani U. A., Buring J. E. (1996) A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA 276, 1147–1151 - PubMed
-
- Evans J. R. (2001) Risk factors for age-related macular degeneration. Prog. Retin. Eye Res 20, 227–253 - PubMed
-
- Khan J. C., Thurlby D. A., Shahid H., Clayton D. G., Yates J. R., Bradley M., Moore A. T., Bird A. C. (2006) Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br. J. Ophthalmol 90, 75–80 - PMC - PubMed
-
- Vingerling J. R., Hofman A., Grobbee D. E., de Jong P. T. (1996) Age-related macular degeneration and smoking. The Rotterdam Study. Arch. Ophthalmol 114, 1193–1196 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

