Development of tumor-reactive T cells after nonmyeloablative allogeneic hematopoietic stem cell transplant for chronic lymphocytic leukemia
- PMID: 19567591
- PMCID: PMC2785487
- DOI: 10.1158/1078-0432.CCR-09-0199
Development of tumor-reactive T cells after nonmyeloablative allogeneic hematopoietic stem cell transplant for chronic lymphocytic leukemia
Abstract
Purpose: Allogeneic nonmyeloablative hematopoietic stem cell transplant (NM-HSCT) can result in durable remission of chronic lymphocytic leukemia (CLL). It is thought that the efficacy of NM-HSCT is mediated by recognition of tumor cells by T cells in the donor stem cell graft. We evaluated the development of CTLs specific for CLL after NM-HSCT to determine if their presence correlated with antitumor efficacy.
Experimental design: Peripheral blood mononuclear cells obtained from 12 transplant recipients at intervals after NM-HSCT were stimulated in vitro with CLL cells. Polyclonal T-cell lines and CD8(+) T-cell clones were derived from these cultures and evaluated for lysis of donor and recipient target cells including CLL. The presence and specificity of responses was correlated with clinical outcomes.
Results: Eight of the 12 patients achieved remission or a major antitumor response and all 8 developed CD8(+) and CD4(+) T cells specific for antigens expressed by CLL. A clonal analysis of the CD8(+) T-cell response identified T cells specific for multiple minor histocompatibility (H) antigens expressed on CLL in six of the responding patients. A significant fraction of the CD8(+) T-cell response in some patients was also directed against nonshared tumor-specific antigens. By contrast, CLL-reactive T cells were not detected in the four patients who had persistent CLL after NM-HSCT, despite the development of graft-versus-host disease.
Conclusions: The development of a diverse T-cell response specific for minor H and tumor-associated antigens expressed by CLL predicts an effective graft-versus-leukemia response after NM-HSCT.
Conflict of interest statement
Conflict-of-interest disclosure: The authors have no relevant conflicts of interest to declare.
Figures

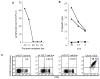
 ) and donor B-LCL (□). Data is shown for an effector to target ratio of 30:1 or 40:1. A. Reactivity of T cell lines generated from patients who had a major antitumor response after NM-HSCT. B. Reactivity of T cell lines generated from patients who had persistent or progressive disease after NM-HSCT.
) and donor B-LCL (□). Data is shown for an effector to target ratio of 30:1 or 40:1. A. Reactivity of T cell lines generated from patients who had a major antitumor response after NM-HSCT. B. Reactivity of T cell lines generated from patients who had persistent or progressive disease after NM-HSCT.

Comment in
-
Revealing tumor immunity after hematopoietic stem cell transplantation.Clin Cancer Res. 2009 Jul 15;15(14):4515-7. doi: 10.1158/1078-0432.CCR-09-0873. Epub 2009 Jul 7. Clin Cancer Res. 2009. PMID: 19584145
References
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. - PubMed
-
- Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–73. - PubMed
-
- McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. - PubMed
-
- Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54. - PubMed
-
- Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

