The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana
- PMID: 19567706
- PMCID: PMC2714930
- DOI: 10.1105/tpc.109.066670
The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana
Abstract
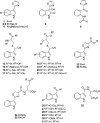
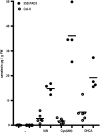
Accumulation of camalexin, the characteristic phytoalexin of Arabidopsis thaliana, is induced by a great variety of plant pathogens. It is derived from Trp, which is converted to indole-3-acetonitrile (IAN) by successive action of the cytochrome P450 enzymes CYP79B2/B3 and CYP71A13. Extracts from wild-type plants and camalexin biosynthetic mutants, treated with silver nitrate or inoculated with Phytophthora infestans, were comprehensively analyzed by ultra-performance liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry. This metabolomics approach was combined with precursor feeding experiments to characterize the IAN metabolic network and to identify novel biosynthetic intermediates and metabolites of camalexin. Indole-3-carbaldehyde and indole-3-carboxylic acid derivatives were shown to originate from IAN. IAN conjugates with glutathione, gamma-glutamylcysteine, and cysteine [Cys(IAN)] accumulated in challenged phytoalexin deficient3 (pad3) mutants. Cys(IAN) rescued the camalexin-deficient phenotype of cyp79b2 cyp79b3 and was itself converted to dihydrocamalexic acid (DHCA), the known substrate of CYP71B15 (PAD3), by microsomes isolated from silver nitrate-treated Arabidopsis leaves. Surprisingly, yeast-expressed CYP71B15 also catalyzed thiazoline ring closure, DHCA formation, and cyanide release with Cys(IAN) as substrate. In conclusion, in the camalexin biosynthetic pathway, IAN is derivatized to the intermediate Cys(IAN), which serves as substrate of the multifunctional cytochrome P450 enzyme CYP71B15.
Figures




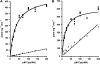


References
-
- Bednarek, P., Pislewska-Bednarek, M., Svatos, A., Schneider, B., Doubsky, J., Mansurova, M., Humphry, M., Consonni, C., Panstruga, R., Sanchez-Vallet, A., Molina, A., and Schulze-Lefert, P. (2009). A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323 101–106. - PubMed
-
- Blum, R., Beck, A., Korte, A., Stengel, A., Letzel, T., Lendzian, K., and Grill, E. (2007). Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 49 740–749. - PubMed
-
- Böttcher, C., von Roepenack-Lahaye, E., Schmidt, J., Schmotz, C., Neumann, S., Scheel, D., and Clemens, S. (2008). Metabolome analysis of biosynthetic mutants reveals a diversity of metabolic changes and allows identification of a large number of new compounds in Arabidopsis. Plant Physiol. 147 2107–2120. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

