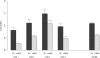Handheld vs. laptop computers for electronic data collection in clinical research: a crossover randomized trial
- PMID: 19567799
- PMCID: PMC2744716
- DOI: 10.1197/jamia.M3041
Handheld vs. laptop computers for electronic data collection in clinical research: a crossover randomized trial
Abstract
Objective: To compare users' speed, number of entry errors and satisfaction in using two current devices for electronic data collection in clinical research: handheld and laptop computers.
Design: The authors performed a randomized cross-over trial using 160 different paper-based questionnaires and representing altogether 45,440 variables. Four data coders were instructed to record, according to a random predefined and equally balanced sequence, the content of these questionnaires either on a laptop or on a handheld computer. Instructions on the kind of device to be used were provided to data-coders in individual sealed and opaque envelopes. Study conditions were controlled and the data entry process performed in a quiet environment.
Measurements: The authors compared the duration of the data recording process, the number of errors and users' satisfaction with the two devices. The authors divided errors into two separate categories, typing and missing data errors. The original paper-based questionnaire was used as a gold-standard.
Results: The overall duration of the recording process was significantly reduced (2.0 versus 3.3 min) when data were recorded on the laptop computer (p < 0.001). Data accuracy also improved. There were 5.8 typing errors per 1,000 entries with the laptop compared to 8.4 per 1,000 with the handheld computer (p < 0.001). The difference was even more important for missing data which decreased from 22.8 to 2.9 per 1,000 entries when a laptop was used (p < 0.001). Users found the laptop easier, faster and more satisfying to use than the handheld computer.
Conclusions: Despite the increasing use of handheld computers for electronic data collection in clinical research, these devices should be used with caution. They double the duration of the data entry process and significantly increase the risk of typing errors and missing data. This may become a particularly crucial issue in studies where these devices are provided to patients or healthcare workers, unfamiliar with computer technologies, for self-reporting or research data collection processes.
Figures
Similar articles
-
Handheld computers for survey and trial data collection in resource-poor settings: development and evaluation of PDACT, a Palm Pilot interviewing system.Int J Med Inform. 2009 Nov;78(11):721-31. doi: 10.1016/j.ijmedinf.2008.10.006. Epub 2009 Jan 20. Int J Med Inform. 2009. PMID: 19157967
-
Comparison of handheld computer-assisted and conventional paper chart documentation of medical records. A randomized, controlled trial.J Bone Joint Surg Am. 2004 Mar;86(3):553-60. doi: 10.2106/00004623-200403000-00014. J Bone Joint Surg Am. 2004. PMID: 14996882 Clinical Trial.
-
Point-of-care technology supports bedside documentation.J Nurs Adm. 2010 Sep;40(9):360-5. doi: 10.1097/NNA.0b013e3181ee4248. J Nurs Adm. 2010. PMID: 20798618
-
A review of randomized controlled trials comparing the effectiveness of hand held computers with paper methods for data collection.BMC Med Inform Decis Mak. 2006 May 31;6:23. doi: 10.1186/1472-6947-6-23. BMC Med Inform Decis Mak. 2006. PMID: 16737535 Free PMC article. Review.
-
Matching point-of care devices to clinicians for positive outcomes.Home Healthc Nurse. 2005 Jul;23(7):452-9; quiz 460-1. doi: 10.1097/00004045-200507000-00011. Home Healthc Nurse. 2005. PMID: 16010145 Review.
Cited by
-
Electronic Records With Tablets at the Point of Care in an Internal Medicine Unit: Before-After Time Motion Study.JMIR Hum Factors. 2022 Feb 10;9(1):e30512. doi: 10.2196/30512. JMIR Hum Factors. 2022. PMID: 35142624 Free PMC article.
-
Implementing school malaria surveys in Kenya: towards a national surveillance system.Malar J. 2010 Oct 30;9:306. doi: 10.1186/1475-2875-9-306. Malar J. 2010. PMID: 21034492 Free PMC article.
-
Assessing the Usability of Six Data Entry Mobile Interfaces for Caregivers: A Randomized Trial.JMIR Hum Factors. 2015 Dec 15;2(2):e15. doi: 10.2196/humanfactors.4093. JMIR Hum Factors. 2015. PMID: 27025648 Free PMC article.
-
A systematic review of healthcare applications for smartphones.BMC Med Inform Decis Mak. 2012 Jul 10;12:67. doi: 10.1186/1472-6947-12-67. BMC Med Inform Decis Mak. 2012. PMID: 22781312 Free PMC article.
-
Feasibility and acceptability of a psychosocial and adherence electronic patient reported outcomes (PROs) system at an HIV care center in southern India.AIDS Care. 2020 May;32(5):630-636. doi: 10.1080/09540121.2019.1668532. Epub 2019 Sep 18. AIDS Care. 2020. PMID: 31533448 Free PMC article.
References
-
- Sahoo U, Bhatt A. Electronic data capture (EDC)—A new mantra for clinical trials Qual Assur 2003;10(3–4):117-121. - PubMed
-
- Schmier JK, Kane DW, Halpern MT. Practical applications of usability theory to electronic data collection for clinical trials Contemp Clin Trials 2005;26(3):376-385. - PubMed
-
- Miller RH, Hillman JM, Given RS. Physician use of IT: Results from the Deloitte Research survey J Healthc Inf Manag 2004;18(1):72-80. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources