Presynaptic targeting of alpha4beta 2 nicotinic acetylcholine receptors is regulated by neurexin-1beta
- PMID: 19567877
- PMCID: PMC2749099
- DOI: 10.1074/jbc.M109.017384
Presynaptic targeting of alpha4beta 2 nicotinic acetylcholine receptors is regulated by neurexin-1beta
Abstract
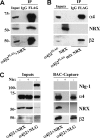
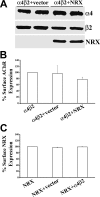
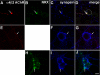
The mechanisms involved in the targeting of neuronal nicotinic acetylcholine receptors (AChRs), critical for their functional organization at neuronal synapses, are not well understood. We have identified a novel functional association between alpha4beta2 AChRs and the presynaptic cell adhesion molecule, neurexin-1beta. In non-neuronal tsA 201 cells, recombinant neurexin-1beta and mature alpha4beta2 AChRs form complexes. alpha4beta2 AChRs and neurexin-1beta also coimmunoprecipitate from rat brain lysates. When exogenous alpha4beta2 AChRs and neurexin-1beta are coexpressed in hippocampal neurons, they are robustly targeted to hemi-synapses formed between these neurons and cocultured tsA 201 cells expressing neuroligin-1, a postsynaptic binding partner of neurexin-1beta. The extent of synaptic targeting is significantly reduced in similar experiments using a mutant neurexin-1beta lacking the extracellular domain. Additionally, when alpha4beta2 AChRs, alpha7 AChRs, and neurexin-1beta are coexpressed in the same neuron, only the alpha4beta2 AChR colocalizes with neurexin-1beta at presynaptic terminals. Collectively, these data suggest that neurexin-1beta targets alpha4beta2 AChRs to presynaptic terminals, which mature by trans-synaptic interactions between neurexins and neuroligins. Interestingly, human neurexin-1 gene dysfunctions have been implicated in nicotine dependence and in autism spectrum disorders. Our results provide novel insights as to possible mechanisms by which dysfunctional neurexins, through downstream effects on alpha4beta2 AChRs, may contribute to the etiology of these neurological disorders.
Figures



References
-
- Wonnacott S. (1997) Trends Neurosci. 20,92–98 - PubMed
-
- Role L. W., Berg D. K. (1996) Neuron 16,1077–1085 - PubMed
-
- Millar N. S. (2003) Biochem. Soc. Trans. 31,869–874 - PubMed
-
- Dani J. A., Bertrand D. (2007) Annu. Rev. Pharmacol. Toxicol. 47,699–729 - PubMed
-
- Picciotto M. R., Caldarone B. J., King S. L., Zachariou V. (2000) Neuropsychopharmacology 22,451–465 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

