Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion
- PMID: 19567882
- PMCID: PMC2738574
- DOI: 10.1182/blood-2009-02-203216
Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion
Abstract
The erythrocyte membrane skeleton is the best understood cytoskeleton. Because its protein components have homologs in virtually all other cells, the membrane serves as a fundamental model of biologic membranes. Modern textbooks portray the membrane as a 2-dimensional spectrin-based membrane skeleton attached to a lipid bilayer through 2 linkages: band 3-ankyrin-beta-spectrin and glycophorin C-protein 4.1-beta-spectrin.(1-7) Although evidence supports an essential role for the first bridge in regulating membrane cohesion, rupture of the glycophorin C-protein 4.1 interaction has little effect on membrane stability.(8) We demonstrate the existence of a novel band 3-adducin-spectrin bridge that connects the spectrin/actin/protein 4.1 junctional complex to the bilayer. As rupture of this bridge leads to spontaneous membrane fragmentation, we conclude that the band 3-adducin-spectrin bridge is important to membrane stability. The required relocation of part of the band 3 population to the spectrin/actin junctional complex and its formation of a new bridge with adducin necessitates a significant revision of accepted models of the erythrocyte membrane.
Figures
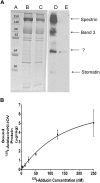

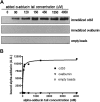
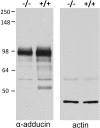
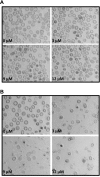


References
-
- Alberts B, Johnson A, Lewis J, Roberts K, Walter P, Baff M. Molecular Biology of the Cell. 5th Ed. New York, NY: Garland Science Publishing; 2008.
-
- Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligshon U. Williams Hematology. 6th Ed. New York, NY: McGraw-Hill Professional; 2000.
-
- Cooper GM, Hausman RE. The Cell: A Molecular Approach. 3rd Ed. Washington, DC: ASM Press; 2004.
-
- Greer JP, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B. Wintrobe's Clinical Hematology. 11th Ed. Baltimore, MD: Lippincott Williams & Wilkins; 2003.
-
- Metzler DE. Biochemistry: The Chemical Reactions of the Living Cells. 2nd Ed. Vol 1. New York, NY: Academic Press; 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

