Using mouse models to understand normal and abnormal urogenital tract development
- PMID: 19568352
- PMCID: PMC2659372
- DOI: 10.4161/org.8173
Using mouse models to understand normal and abnormal urogenital tract development
Abstract
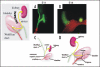
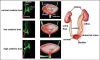
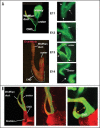
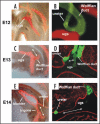
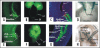
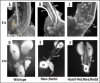
Removal of toxic substances from the blood depends on patent connections between the kidneys, ureters and bladder that are established when the ureter is transposed from its original insertion site in the Wolffian duct, to the bladder, its final insertion site. The Ureteral Bud Theory of Mackie and Stephens suggests that repositioning of the ureter orifice occurs as the trigone forms from the common nephric duct (CND), the caudal-most Wolffian duct segment. According to this model, insertion of the CND into the bladder and its expansion into the trigone both repositions the ureter in the bladder and enables it to separate from the Wolffian duct. The availability of new mouse models has enabled to re-examine this hypothesis using morphological analysis and lineage studies to follow the fate of the ureter and CND during the maturation process. We find that in contrast to what has been previously thought, the CND does not differentiate into the trigone but instead, undergoes apoptosis, a step that enables the ureter to separate from the Wolffian duct. Apoptosis occurs as the CND and ureter merge with the urogenital sinus positioning the ureter orifice at a site close to the Wolffian duct. Finally, expansion of the bladder moves the ureter orifice which is now fused with epithelium to its final position which is at the bladder neck. Interestingly, CND apoptosis appears to depend on close proximity to the bladder, suggesting that the bladder may be a source of signals that induce cell death. Together, these studies provide new insights into the normal process of ureter maturation, and shed light on possible causes of obstruction and reflux, ureteral abnormalities that affect 1-2% of the human population.
Keywords: apoptosis; bladder; common nephric duct; development; obstruction; reflux; ureter maturation; urinary tract.
Figures

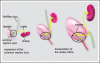



References
-
- Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol. 1975;114:274–280. - PubMed
-
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 2005;37:1082–1089. - PubMed
-
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. - PubMed
-
- Alcaraz A, Vinaixa F, Tejedo-Mateu A, Fores MM, Gotzens V, Mestres CA, et al. Obstruction and recanalization of the ureter during embryonic development. J Urol. 1991;145:410–416. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
