Sterols and membrane dynamics
- PMID: 19568799
- PMCID: PMC2698314
- DOI: 10.1007/s12154-008-0010-6
Sterols and membrane dynamics
Abstract
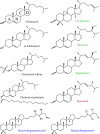
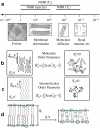
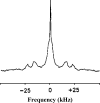
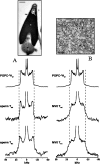
The effect of sterols from mammals, plants, fungi, and bacteria on model and natural membrane dynamics are reviewed, in the frame of ordering-disordering properties of membranes. It is shown that all sterols share a common property: the ability to regulate dynamics in order to maintain membranes in a microfluid state where it can convey important biological processes. Depending on the sterol class, this property is modulated by molecular modifications that have occurred during evolution. The role of sterols in rafts, antibiotic complexes, and in protecting membranes from the destructive action of amphipathic toxins is also discussed.
Figures









References
-
- Ribeiro N, Streiff S, Heissler D, Elhabiri M, Albrecht-Gary AM, Atsumi M, Gotoh M, Desaubry L, Nakatani Y, Ourisson G. Reinforcing effect of bi- and tri-cyclopolyprenols on ‘primitive’ membranes made of polyprenyl phosphates. Tetrahedron. 2007;63:3395–3407. doi: 10.1016/j.tet.2007.01.076. - DOI
-
- Saito H, Suzuki N. Distributions and sources of hopanes, hopanoic acids and hopanols in Miocene to recent sediments from ODP Leg 190, Nankai Trough. Org Geochem. 2007;38:1715–1728. doi: 10.1016/j.orggeochem.2007.05.012. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

