Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: results of a randomised phase III trial
- PMID: 19568958
- PMCID: PMC2724642
- DOI: 10.1007/s11136-009-9493-z
Quality of life of palliative chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction treated with irinotecan combined with 5-fluorouracil and folinic acid: results of a randomised phase III trial
Abstract
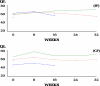
Purpose: The quality of life (QL) of advanced gastric cancer patients receiving irinotecan, folinic acid and 5-fluorouracil (5-FU) (IF arm) or cisplatin with 5-FU (CF arm) is presented.
Methods: Patients with measurable or evaluable advanced gastric cancer received IF weekly for 6/7 weeks or CF q4 weeks. QL was assessed using the EORTC QLQ-C30 at baseline, subsequently every 8 weeks until progression and thereafter every 3 months until death. The QL data were analysed using several statistical methods including summary measures and pattern-mixture modelling.
Results: A total of 333 patients were randomised and treated (IF 170, CF 163). The time-to-progression for IF and CF was 5.0 and 4.2 months (P = 0.088), respectively. The overall compliance rates for QL questionnaire completion were 60 and 56% in the IF and CF arms, respectively. Significant treatment differences were observed for the physical functioning scale (P = 0.024), nausea\vomiting (P = 0.001) and EQ-5D thermometer (P = 0.020) in favour of the IF treatment arm.
Conclusion: There was a trend in favour of IF over CF in time-to-progression. The IF group also demonstrated a better safety profile than CF and a better QL on a number of multi-item scales, suggesting that IF offers an alternative first-line platinum-free treatment option for advanced gastric cancer.
Figures
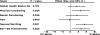
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/(SICI)1097-0215(19990924)83:1<18::AID-IJC5>3.0.CO;2-M', 'is_inner': False, 'url': 'https://doi.org/10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m'}, {'type': 'PubMed', 'value': '10449602', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10449602/'}]}
- Pisani, P., Parkin, D. M., Bray, F., & Ferlay, J. (1999). Estimates of the worldwide mortality from 25 cancers in 1990. International Journal of Cancer,83(1), 18–29. doi:10.1002/(SICI)1097-0215(19990924)83:1<18::AID-IJC5>3.0.CO;2-M. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.3322/canjclin.55.1.10', 'is_inner': False, 'url': 'https://doi.org/10.3322/canjclin.55.1.10'}, {'type': 'PubMed', 'value': '15661684', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15661684/'}]}
- Jemal, A., Murray, T., Ward, E., Samuels, A., Tiwari, R. C., Ghafoor, A., et al. (2005). Cancer statistics, 2005. CA: A Cancer Journal for Clinicians,55(1), 10–30. doi:10.3322/canjclin.55.1.10. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1097/00042737-200403000-00003', 'is_inner': False, 'url': 'https://doi.org/10.1097/00042737-200403000-00003'}, {'type': 'PubMed', 'value': '15195888', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15195888/'}]}
- Dickson, J. L., & Cunningham, D. (2004). Systemic treatment of gastric cancer. European Journal of Gastroenterology and Hepatology,16(3), 255–263. doi:10.1097/00042737-200403000-00003. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '12810456', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12810456/'}]}
- Bugat, R. (2003). Irinotecan in the treatment of gastric cancer. Annals of Oncology,14(Suppl 2), S37–S40. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1634/theoncologist.9-suppl_2-9', 'is_inner': False, 'url': 'https://doi.org/10.1634/theoncologist.9-suppl_2-9'}, {'type': 'PubMed', 'value': '15161986', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15161986/'}]}
- Van Cutsem, E. (2004). The treatment of advanced gastric cancer: New findings on the activity of the taxanes. The Oncologist,9(Suppl 2), S9–S15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

