Solution-phase parallel synthesis of a multi-substituted benzo[b]thiophene library
- PMID: 19569714
- PMCID: PMC2743771
- DOI: 10.1021/cc9000604
Solution-phase parallel synthesis of a multi-substituted benzo[b]thiophene library
Abstract
Generation of a library using parallel syntheses of multi-substituted benzo[b]thiophenes is described. The requisite 3-iodobenzo[b]thiophenes are readily prepared in excellent yields from various alkynes bearing electron-rich aromatic rings by electrophilic cyclization using I(2) in CH(2)Cl(2). The heteroaromatic carbon-iodine bonds allow further diversification by palladium-catalyzed Suzuki-Miyaura, Sonogashira, Heck, and carboalkoxylation chemistry to give multi-substituted benzo[b]thiophene derivatives.
Figures


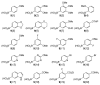

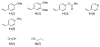

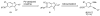
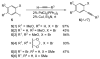


References
-
- An HY, Cook PD. Chem. Rev. 2000;100:3311–3340. - PubMed
-
- Sun CM. Comb. Chem. High Throughput Screening. 1999;2:299–318. - PubMed
-
- Merritt AT. Comb. Chem. High Throughput Screening. 1998;1:57–72. - PubMed
-
- Goodnow RA, Jr., Guba W, Haap W. Comb. Chem. High Throughput Screening. 2003;6:649–660. - PubMed
-
- Schuffenhauer A, Popov M, Schopfer U, Acklin P, Stanek J, Jacoby EC. Comb. Chem. High Throughput Screening. 2004;7:771–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

