Microscale recording from human motor cortex: implications for minimally invasive electrocorticographic brain-computer interfaces
- PMID: 19569885
- PMCID: PMC2875251
- DOI: 10.3171/2009.4.FOCUS0980
Microscale recording from human motor cortex: implications for minimally invasive electrocorticographic brain-computer interfaces
Abstract
Object: There is a growing interest in the use of recording from the surface of the brain, known as electrocorticography (ECoG), as a practical signal platform for brain-computer interface application. The signal has a combination of high signal quality and long-term stability that may be the ideal intermediate modality for future application. The research paradigm for studying ECoG signals uses patients requiring invasive monitoring for seizure localization. The implanted arrays span cortex areas on the order of centimeters. Currently, it is unknown what level of motor information can be discerned from small regions of human cortex with microscale ECoG recording.
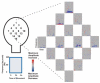
Methods: In this study, a patient requiring invasive monitoring for seizure localization underwent concurrent implantation with a 16-microwire array (1-mm electrode spacing) placed over primary motor cortex. Microscale activity was recorded while the patient performed simple contra- and ipsilateral wrist movements that were monitored in parallel with electromyography. Using various statistical methods, linear and nonlinear relationships between these microcortical changes and recorded electromyography activity were defined.
Results: Small regions of primary motor cortex (< 5 mm) carry sufficient information to separate multiple aspects of motor movements (that is, wrist flexion/extension and ipsilateral/contralateral movements).
Conclusions: These findings support the conclusion that small regions of cortex investigated by ECoG recording may provide sufficient information about motor intentions to support brain-computer interface operations in the future. Given the small scale of the cortical region required, the requisite implanted array would be minimally invasive in terms of surgical placement of the electrode array.
Figures



References
-
- Bjornsson CS, Oh SJ, Al-Kofahi YA, Lim YJ, Smith KL, Turner JN, et al. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J Neural Eng. 2006;3:196–207. - PubMed
-
- Blakely T, Miller KJ, Rao RP, Holmes MD, Ojemann JG. Localization and classification of phonemes using high spatial resolution electrocorticography (ECoG) grids. Conf Proc IEEE Eng Med Biol Soc; 2008. pp. 4964–4967. 2008. - PubMed
-
- Boulton AA, Baker GB, Vanderwolf CH. Neurophysiological Techniques II: Applications to Neural Systems. Humana Press; Totowa. New Jersey: 1990.
-
- Cover TM, Thomas JA. Elements of Information Theory. John Wiley & Sons; New York: 1991.
-
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

