Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies
- PMID: 19569968
- PMCID: PMC2861951
- DOI: 10.1089/hum.2009.094
Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies
Abstract
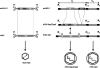
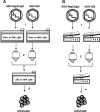
The ability of recombinant adeno-associated viral (rAAV) vectors to exhibit minimal immunogenicity and little to no toxicity or inflammation while eliciting robust, multiyear gene expression in vivo are only a few of the salient features that make them ideally suited for many gene therapy applications. A major hurdle for the use of rAAV in sizeable research and clinical applications is the lack of efficient and versatile large-scale production systems. Continued progression toward flexible, scalable production techniques is a prerequisite to support human clinical evaluation of these novel biotherapeutics. This review examines the current state of large-scale production methods that employ the herpes simplex virus type 1 (HSV) platform to produce rAAV vectors for gene delivery. Improvements have substantially advanced the HSV/AAV hybrid method for large-scale rAAV manufacture, facilitating the generation of highly potent, clinical-grade purity rAAV vector stocks. At least one human clinical trial employing rAAV generated via rHSV helper-assisted replication is poised to commence, highlighting the advances and relevance of this production method.
Figures
References
-
- Aucoin M.G. Perrier M. Kamen A.A. Critical assessment of current adeno-associated viral vector production and quantification methods. Biotechnol. Adv. 2008;26:73–88. - PubMed
-
- Blankinship M.J. Gregorevic P. Chamberlain J.S. Gene therapy strategies for Duchenne muscular dystrophy utilizing recombinant adeno-associated virus vectors. Mol. Ther. 2006;13:241–249. - PubMed
-
- Blits B. Bunge M.B. Direct gene therapy for repair of the spinal cord. J. Neurotrauma. 2006;23:508–520. - PubMed
-
- Bloch J. Bachoud-Levi A.C. Deglon N. Lefaucheur J.P. Winkel L. Palfi S. Nguyen J.P. Bourdet C. Gaura V. Remy P. Brugieres P. Boisse M.F. Baudic S. Cesaro P. Hantraye P. Aebischer P. Peschanski M. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: Results of a phase I study. Hum. Gene Ther. 2004;15:968–975. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

