Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF
- PMID: 19570028
- PMCID: PMC2695583
- DOI: 10.1042/AN20090002
Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF
Abstract
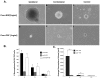
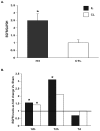
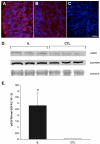
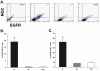
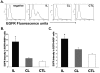
There is an increase in the numbers of neural precursors in the SVZ (subventricular zone) after moderate ischaemic injuries, but the extent of stem cell expansion and the resultant cell regeneration is modest. Therefore our studies have focused on understanding the signals that regulate these processes towards achieving a more robust amplification of the stem/progenitor cell pool. The goal of the present study was to evaluate the role of the EGFR [EGF (epidermal growth factor) receptor] in the regenerative response of the neonatal SVZ to hypoxic/ischaemic injury. We show that injury recruits quiescent cells in the SVZ to proliferate, that they divide more rapidly and that there is increased EGFR expression on both putative stem cells and progenitors. With the amplification of the precursors in the SVZ after injury there is enhanced sensitivity to EGF, but not to FGF (fibroblast growth factor)-2. EGF-dependent SVZ precursor expansion, as measured using the neurosphere assay, is lost when the EGFR is pharmacologically inhibited, and forced expression of a constitutively active EGFR is sufficient to recapitulate the exaggerated proliferation of the neural stem/progenitors that is induced by hypoxic/ischaemic brain injury. Cumulatively, our results reveal that increased EGFR signalling precedes that increase in the abundance of the putative neural stem cells and our studies implicate the EGFR as a key regulator of the expansion of SVZ precursors in response to brain injury. Thus modulating EGFR signalling represents a potential target for therapies to enhance brain repair from endogenous neural precursors following hypoxic/ischaemic and other brain injuries.
Figures

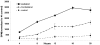



References
-
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KCK, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
