TAp63 prevents premature aging by promoting adult stem cell maintenance
- PMID: 19570515
- PMCID: PMC3418222
- DOI: 10.1016/j.stem.2009.04.003
TAp63 prevents premature aging by promoting adult stem cell maintenance
Abstract
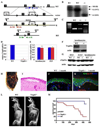
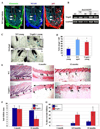
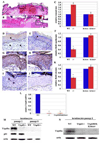
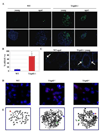
The cellular mechanisms that regulate the maintenance of adult tissue stem cells are still largely unknown. We show here that the p53 family member, TAp63, is essential for maintenance of epidermal and dermal precursors and that, in its absence, these precursors senesce and skin ages prematurely. Specifically, we have developed a TAp63 conditional knockout mouse and used it to ablate TAp63 in the germline (TAp63(-/-)) or in K14-expressing cells in the basal layer of the epidermis (TAp63(fl/fl);K14cre+). TAp63(-/-) mice age prematurely and develop blisters, skin ulcerations, senescence of hair follicle-associated dermal and epidermal cells, and decreased hair morphogenesis. These phenotypes are likely due to loss of TAp63 in dermal and epidermal precursors since both cell types show defective proliferation, early senescence, and genomic instability. These data indicate that TAp63 serves to maintain adult skin stem cells by regulating cellular senescence and genomic stability, thereby preventing premature tissue aging.
Figures



Comment in
-
SKP-ing TAp63: stem cell depletion, senescence, and premature aging.Cell Stem Cell. 2009 Jul 2;5(1):1-2. doi: 10.1016/j.stem.2009.06.015. Cell Stem Cell. 2009. PMID: 19570504
References
-
- Ackerman AB. Histologic Diagnosis of Inflammatory Skin Diseases. 2nd Ed. Baltimore, MD: Williams & Wilkins; 1997.
-
- Beretta C, Chiarelli A, Testoni B, Mantovani R, Guerrini L. Regulation of the cyclin-dependent kinase inhibitor p57Kip2 expression by p63. Cell Cycle. 2005;4:1625–1631. - PubMed
-
- Biernaskie JA, McKenzie IA, Toma JG, Miller FD. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat Protoc. 2006;1:2803–2812. - PubMed
-
- Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death and Differentiation. 2006;13:1037–1047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

