Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes
- PMID: 19570823
- PMCID: PMC2739219
- DOI: 10.4049/jimmunol.0803987
Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes
Abstract
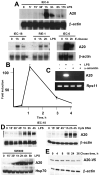
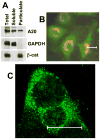
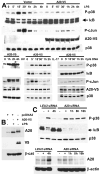
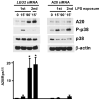
Although enterocytes are capable of innate immune responses, the intestinal epithelium is normally tolerant to commensal bacteria. To elucidate the mechanisms of tolerance, we examined the effect of preexposure to LPS on activation of p38, c-Jun, and NF-kappaB in enterocytes by several inflammatory and stress stimuli. Shortly after the initial LPS challenge, enterocytes become tolerant to restimulation with LPS or CpG DNA, but not with IL-17 or UV. The state of tolerance, which lasts 20-26 h, temporally coincides with LPS-induced expression of the anti-inflammatory ubiquitin-editing enzyme A20. Small interfering RNA silencing of A20 prevents tolerance, whereas ectopic expression of A20 blocks responses to LPS and CpG DNA, but not to IL-17 or UV. A20 levels in the epithelium of the small intestine are low at birth and following gut decontamination with antibiotics, but high under conditions of bacterial colonization. In the small intestine of adult rodents, A20 prominently localizes to the luminal interface of villus enterocytes. Lower parts of the crypts display relatively low levels of A20, but relatively high levels of phospho-p38. Gut decontamination with antibiotics reduces the levels of both A20 and phospho-p38. Along with the fact that A20-deficient mice develop severe intestinal inflammation, our results indicate that induction of A20 plays a key role in the tolerance of the intestinal epithelium to TLR ligands and bacteria.
Conflict of interest statement
Figures

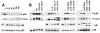



References
-
- Fukata M, Abreu MT. TLR4 signalling in the intestine in health and disease. Biochem Soc Trans. 2007;35:1473–1478. - PubMed
-
- Lee J, Mo JH, Shen C, Rucker AN, Raz E. Toll-like receptor signaling in intestinal epithelial cells contributes to colonic homoeostasis. Curr Opin Gastroenterol. 2007;23:27–31. - PubMed
-
- Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Curr Opin Hematol. 2007;14:48–54. - PubMed
-
- Rakoff-Nahoum S, Medzhitov R. Role of the innate immune system and host-commensal mutualism. Curr Top Microbiol Immunol. 2006;308:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

