20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism
- PMID: 19570883
- PMCID: PMC2739708
- DOI: 10.1152/ajprenal.00146.2009
20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism
Abstract
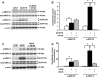
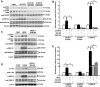
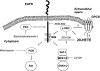
20-Hydroxyeicosatetraenoic acid (20-HETE) has been reported to promote mitogenicity in a variety of cell types, including renal epithelial cells. However, the signal transduction pathways activated by 20-HETE have not been fully defined. The present study evaluated the effects of 20-HETE and its more stable agonist analogs 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-20-HEDE) and N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-20-HEDGE) on the Raf/MEK/ERK and phosphatidylinositol 3-kinase (PI3K)-Akt pathway in LLC-PK(1) renal epithelial cells. 20-HETE (20 microM) increased phosphorylation of Raf-1 (2.5 +/- 0.2-fold), MEK1/2 (6.3 +/- 1.6-fold), and ERK1/2 (5.8 +/- 0.3-fold) compared with vehicle-treated cells. Similarly, the 20-HETE analogs also strongly activated ERK1/2 in a Raf-1- and MEK1/2-dependent manner. Moreover, 5,14-20-HEDE increased Akt phosphorylation by 2.2 +/- 0.3-fold. 20-HETE and 5,14-20-HEDE also promoted activation (Y1086) of epidermal growth factor receptor (EGFR; Y1086) by 1.9 +/- 0.2- and 2.5 +/- 0.2-fold, respectively. These effects were completely blocked by the EGFR inhibitor EKB-569 (0.1 microM). Moreover, EKB-569 (0.1 microM), as well as a c-Src inhibitor, SKI-606 (0.05 microM), completely abolished the 20-HETE-mediated activation of the Raf/MEK/ERK and PI3K-Akt pathways. Blockade of PKC with bisindolylmaleimide I had no effect on 20-HETE-induced ERK1/2 activation. This study demonstrated that 20-HETE activated the Raf/MEK/ERK and Akt pathways in renal epithelial cells secondary to the activation of c-Src and EGFR.
Figures





References
-
- Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol Renal Physiol 277: F790–F796, 1999. - PubMed
-
- Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, Tischler M, Arndt K, Discafani C, Etienne C, Gibbons J, Grod J, Lucas J, Weber JM, Boschelli F. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem 44: 3965–3977, 2001. - PubMed
-
- Chen Y, Medhora M, Falck JR, Pritchard KA Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 291: L378–L385, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

