Regulation of fission yeast myosin-II function and contractile ring dynamics by regulatory light-chain and heavy-chain phosphorylation
- PMID: 19570908
- PMCID: PMC2735492
- DOI: 10.1091/mbc.e09-04-0346
Regulation of fission yeast myosin-II function and contractile ring dynamics by regulatory light-chain and heavy-chain phosphorylation
Abstract
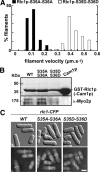
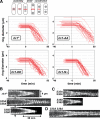
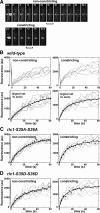
We investigated the role of regulatory light-chain (Rlc1p) and heavy-chain phosphorylation in controlling fission yeast myosin-II (Myo2p) motor activity and function during cytokinesis. Phosphorylation of Rlc1p leads to a fourfold increase in Myo2p's in vitro motility rate, which ensures effective contractile ring constriction and function. Surprisingly, unlike with smooth muscle and nonmuscle myosin-II, RLC phosphorylation does not influence the actin-activated ATPase activity of Myo2p. A truncated form of Rlc1p lacking its extended N-terminal regulatory region (including phosphorylation sites) supported maximal Myo2p in vitro motility rates and normal contractile ring function. Thus, the unphosphorylated N-terminal extension of Rlc1p can uncouple the ATPase and motility activities of Myo2p. We confirmed the identity of one out of two putative heavy-chain phosphorylation sites previously reported to control Myo2p function and cytokinesis. Although in vitro studies indicated that phosphorylation at Ser-1444 is not needed for Myo2p motor activity, phosphorylation at this site promotes the initiation of contractile ring constriction.
Figures





Similar articles
-
Interactions of Cdc4p, a myosin light chain, with IQ-domain containing proteins in Schizosaccharomyces pombe.Cell Struct Funct. 2001 Dec;26(6):555-65. doi: 10.1247/csf.26.555. Cell Struct Funct. 2001. PMID: 11942609
-
Tropomyosin and myosin-II cellular levels promote actomyosin ring assembly in fission yeast.Mol Biol Cell. 2010 Mar 15;21(6):989-1000. doi: 10.1091/mbc.e09-10-0852. Epub 2010 Jan 28. Mol Biol Cell. 2010. PMID: 20110347 Free PMC article.
-
Fission yeast myosin Myo2 is down-regulated in actin affinity by light chain phosphorylation.Proc Natl Acad Sci U S A. 2017 Aug 29;114(35):E7236-E7244. doi: 10.1073/pnas.1703161114. Epub 2017 Aug 14. Proc Natl Acad Sci U S A. 2017. PMID: 28808035 Free PMC article.
-
Cytokinesis: new roles for myosin.Curr Biol. 2005 Apr 26;15(8):R310-1. doi: 10.1016/j.cub.2005.04.008. Curr Biol. 2005. PMID: 15854900 Review.
-
Myosin-cell wall interactions during cytokinesis in fission yeast: a framework for understanding plant cytokinesis?Cell Biol Int. 2003;27(3):239-40. doi: 10.1016/s1065-6995(02)00311-6. Cell Biol Int. 2003. PMID: 12681321 Review. No abstract available.
Cited by
-
Dividing the spoils of growth and the cell cycle: The fission yeast as a model for the study of cytokinesis.Cytoskeleton (Hoboken). 2011 Feb;68(2):69-88. doi: 10.1002/cm.20500. Cytoskeleton (Hoboken). 2011. PMID: 21246752 Free PMC article. Review.
-
Fission yeast Myo2: Molecular organization and diffusion in the cytoplasm.Cytoskeleton (Hoboken). 2018 Apr;75(4):164-173. doi: 10.1002/cm.21425. Epub 2017 Dec 14. Cytoskeleton (Hoboken). 2018. PMID: 29205883 Free PMC article.
-
Myosin turnover controls actomyosin contractile instability.Proc Natl Acad Sci U S A. 2022 Oct 25;119(43):e2211431119. doi: 10.1073/pnas.2211431119. Epub 2022 Oct 20. Proc Natl Acad Sci U S A. 2022. PMID: 36264833 Free PMC article.
-
Myosin II regulatory light chain phosphorylation and formin availability modulate cytokinesis upon changes in carbohydrate metabolism.Elife. 2023 Feb 24;12:e83285. doi: 10.7554/eLife.83285. Elife. 2023. PMID: 36825780 Free PMC article.
-
Three's company: the fission yeast actin cytoskeleton.Trends Cell Biol. 2011 Mar;21(3):177-87. doi: 10.1016/j.tcb.2010.11.001. Epub 2010 Dec 7. Trends Cell Biol. 2011. PMID: 21145239 Free PMC article. Review.
References
-
- Andreev O. A., Saraswat L. D., Lowey S., Slaughter C., Borejdo J. Interaction of the N-terminus of chicken skeletal essential light chain 1 with F-actin. Biochemistry. 1999;38:2480–2485. - PubMed
-
- Bahler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. - PubMed
-
- Bezanilla M., Wilson J. M., Pollard T. D. Fission yeast myosin-II isoforms assemble into contractile rings at distinct times during mitosis. Curr. Biol. 2000;10:397–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

