Control of heart rate by cAMP sensitivity of HCN channels
- PMID: 19570998
- PMCID: PMC2715536
- DOI: 10.1073/pnas.0810332106
Control of heart rate by cAMP sensitivity of HCN channels
Abstract
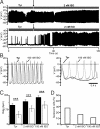
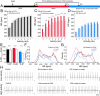
"Pacemaker" f-channels mediating the hyperpolarization-activated nonselective cation current I(f) are directly regulated by cAMP. Accordingly, the activity of f-channels increases when cellular cAMP levels are elevated (e.g., during sympathetic stimulation) and decreases when they are reduced (e.g., during vagal stimulation). Although these biophysical properties seem to make f-channels ideal molecular targets for heart rate regulation by the autonomic nervous system, the exact contribution of the major I(f)-mediating cardiac isoforms HCN2 and HCN4 to sinoatrial node (SAN) function remains highly controversial. To directly investigate the role of cAMP-dependent regulation of hyperpolarization activated cyclic nucleotide activated (HCN) channels in SAN activity, we generated mice with heart-specific and inducible expression of a human HCN4 mutation (573X) that abolishes the cAMP-dependent regulation of HCN channels. We found that hHCN4-573X expression causes elimination of the cAMP sensitivity of I(f) and decreases the maximum firing rates of SAN pacemaker cells. In conscious mice, hHCN4-573X expression leads to a marked reduction in heart rate at rest and during exercise. Despite the complete loss of cAMP sensitivity of I(f), the relative extent of SAN cell frequency and heart rate regulation are preserved. Our data demonstrate that cAMP-mediated regulation of I(f) determines basal and maximal heart rates but does not play an indispensable role in heart rate adaptation during physical activity. Our data also reveal the pathophysiologic mechanism of hHCN4-573X-linked SAN dysfunction in humans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


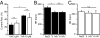
References
-
- Lamas GA, et al. The mode selection trial (MOST) in sinus node dysfunction: Design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J. 2000;140:541–551. - PubMed
-
- Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am Heart J. 1993;125:1148–1154. - PubMed
-
- Fox K, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. - PubMed
-
- Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008;77:274–284. - PubMed
-
- Mangoni M, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

