A tethering mechanism for length control in a processive carbohydrate polymerization
- PMID: 19571009
- PMCID: PMC2715488
- DOI: 10.1073/pnas.0901407106
A tethering mechanism for length control in a processive carbohydrate polymerization
Abstract
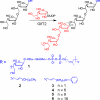
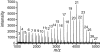
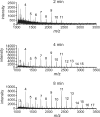
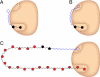
Carbohydrate polymers are the most abundant organic substances on earth. Their degrees of polymerization range from tens to thousands of units, yet polymerases generate the relevant lengths without the aid of a template. To gain insight into template-independent length control, we investigated how the mycobacterial galactofuranosyl-transferase GlfT2 mediates formation of the galactan, a polymer of galactofuranose residues that is an integral part of the cell wall. We show that isolated recombinant GlfT2 can catalyze the synthesis of polymers with degrees of polymerization that are commensurate with values observed in mycobacteria, indicating that length control by GlfT2 is intrinsic. Investigations using synthetic substrates reveal that GlfT2 is processive. The data indicate that GlfT2 controls length by using a substrate tether, which is distal from the site of elongation. The strength of interaction of that tether with the polymerase influences the length of the resultant polymer. Thus, our data identify a mechanism for length control by a template-independent polymerase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
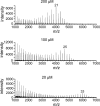
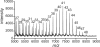
Similar articles
-
Monitoring processivity and length control of a carbohydrate polymerase.J Am Chem Soc. 2011 Aug 17;133(32):12758-66. doi: 10.1021/ja204448t. Epub 2011 Jul 25. J Am Chem Soc. 2011. PMID: 21739979 Free PMC article.
-
Synthetic UDP-galactofuranose analogs reveal critical enzyme-substrate interactions in GlfT2-catalyzed mycobacterial galactan assembly.Org Biomol Chem. 2012 May 28;10(20):4074-87. doi: 10.1039/c2ob25159k. Epub 2012 Apr 13. Org Biomol Chem. 2012. PMID: 22499274
-
Chemical Insight into the Mechanism and Specificity of GlfT2, a Bifunctional Galactofuranosyltransferase from Mycobacteria.J Org Chem. 2016 Sep 16;81(18):8123-30. doi: 10.1021/acs.joc.6b01501. Epub 2016 Aug 30. J Org Chem. 2016. PMID: 27557056 Review.
-
Galactosyl transferases in mycobacterial cell wall synthesis.J Bacteriol. 2008 Feb;190(3):1141-5. doi: 10.1128/JB.01326-07. Epub 2007 Nov 30. J Bacteriol. 2008. PMID: 18055597 Free PMC article.
-
Recent advances toward the inhibition of mAG and LAM synthesis in Mycobacterium tuberculosis.Med Res Rev. 2010 Mar;30(2):290-326. doi: 10.1002/med.20190. Med Res Rev. 2010. PMID: 20099253 Review.
Cited by
-
Trapping of intermediates with substrate analog HBOCoA in the polymerizations catalyzed by class III polyhydroxybutyrate (PHB) synthase from Allochromatium vinosum.ACS Chem Biol. 2015 May 15;10(5):1330-1339. doi: 10.1021/cb5009958. Epub 2015 Feb 25. ACS Chem Biol. 2015. PMID: 25686368 Free PMC article.
-
Two Arabidopsis proteins synthesize acetylated xylan in vitro.Plant J. 2014 Oct;80(2):197-206. doi: 10.1111/tpj.12643. Epub 2014 Sep 20. Plant J. 2014. PMID: 25141999 Free PMC article.
-
Chitin Translocation Is Functionally Coupled with Synthesis in Chitin Synthase.Int J Mol Sci. 2024 Oct 30;25(21):11667. doi: 10.3390/ijms252111667. Int J Mol Sci. 2024. PMID: 39519219 Free PMC article.
-
Monitoring processivity and length control of a carbohydrate polymerase.J Am Chem Soc. 2011 Aug 17;133(32):12758-66. doi: 10.1021/ja204448t. Epub 2011 Jul 25. J Am Chem Soc. 2011. PMID: 21739979 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2009-2010.Mass Spectrom Rev. 2015 May-Jun;34(3):268-422. doi: 10.1002/mas.21411. Epub 2014 May 26. Mass Spectrom Rev. 2015. PMID: 24863367 Free PMC article. Review.
References
-
- Somerville C. Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol. 2006;22:53–78. - PubMed
-
- Stern R. Devising a pathway for hyaluronan catabolism: Are we there yet? Glycobiology. 2003;13:105R–115R. - PubMed
-
- Weigel PH, DeAngelis PL. Hyaluronan synthases: A decade-plus of novel glycosyltransferases. J Biol Chem. 2007;282:36777–36781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

