Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure
- PMID: 19571138
- PMCID: PMC2733220
- DOI: 10.1523/JNEUROSCI.0378-09.2009
Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure
Abstract
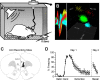
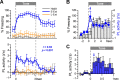
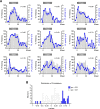
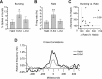
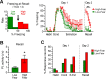
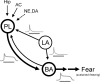
During auditory fear conditioning, it is well established that lateral amygdala (LA) neurons potentiate their response to the tone conditioned stimulus, and that this potentiation is required for conditioned fear behavior. Conditioned tone responses in LA, however, last only a few hundred milliseconds and cannot be responsible for sustained fear responses to a tone lasting tens of seconds. Recent evidence from inactivation and stimulation studies suggests that the prelimbic (PL) prefrontal cortex is necessary for expression of learned fears, but the timing of PL tone responses and correlations with fear behavior have not been studied. Using multichannel unit recording techniques in behaving rats, we observed sustained conditioned tone responses in PL that were correlated with freezing behavior on a second-to-second basis during the presentation of a 30 s tone. PL tone responses were also correlated with conditioned freezing across different experimental phases (habituation, conditioning, extinction). Moreover, the persistence of PL responses after extinction training was associated with failure to express extinction memory. Together with previous inactivation findings, the present results suggest that PL transforms transient amygdala inputs to a sustained output that drives conditioned fear responses and gates the expression of extinction. Given the relatively long latency of conditioned responses we observed in PL (approximately 100 ms after tone onset), we propose that PL integrates inputs from the amygdala, hippocampus, and other cortical sources to regulate the expression of fear memories.
Figures

Comment in
-
Prelimbic prefrontal neurons drive fear expression: a clue for extinction--reconsolidation interactions.J Neurosci. 2009 Oct 28;29(43):13432-4. doi: 10.1523/JNEUROSCI.4299-09.2009. J Neurosci. 2009. PMID: 19864555 Free PMC article. No abstract available.
References
-
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. - PubMed
-
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
