Bistability and non-Gaussian fluctuations in spontaneous cortical activity
- PMID: 19571142
- PMCID: PMC6665653
- DOI: 10.1523/JNEUROSCI.0754-09.2009
Bistability and non-Gaussian fluctuations in spontaneous cortical activity
Abstract
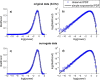
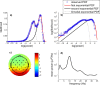
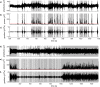
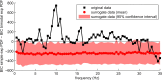
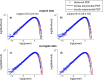
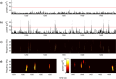
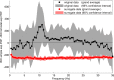
The brain is widely assumed to be a paradigmatic example of a complex, self-organizing system. As such, it should exhibit the classic hallmarks of nonlinearity, multistability, and "nondiffusivity" (large coherent fluctuations). Surprisingly, at least at the very large scale of neocortical dynamics, there is little empirical evidence to support this, and hence most computational and methodological frameworks for healthy brain activity have proceeded very reasonably from a purely linear and diffusive perspective. By studying the temporal fluctuations of power in human resting-state electroencephalograms, we show that, although these simple properties may hold true at some temporal scales, there is strong evidence for bistability and nondiffusivity in key brain rhythms. Bistability is manifest as nonclassic bursting between high- and low-amplitude modes in the alpha rhythm. Nondiffusivity is expressed through the irregular appearance of high amplitude "extremal" events in beta rhythm power fluctuations. The statistical robustness of these observations was confirmed through comparison with Gaussian-rendered phase-randomized surrogate data. Although there is a good conceptual framework for understanding bistability in cortical dynamics, the implications of the extremal events challenge existing frameworks for understanding large-scale brain systems.
Figures



References
-
- Allen PJ, Polizzi G, Krakow K, Fish DR, Lemieux L. Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for subtraction. Neuroimage. 1998;8:229–239. - PubMed
-
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–239. - PubMed
-
- Amari S, Nakahara H, Wu S, Sakai Y. Synchronous firing and higher-order interactions in neuron pool. Neural Comput. 2003;15:127–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
