Abscisic acid has a key role in modulating diverse plant-pathogen interactions
- PMID: 19571312
- PMCID: PMC2719142
- DOI: 10.1104/pp.109.137943
Abscisic acid has a key role in modulating diverse plant-pathogen interactions
Abstract
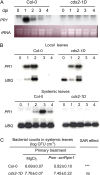
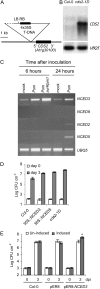
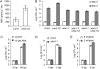
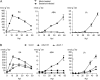
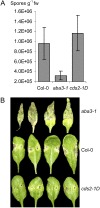
We isolated an activation-tagged Arabidopsis (Arabidopsis thaliana) line, constitutive disease susceptibility2-1D (cds2-1D), that showed enhanced bacterial growth when challenged with various Pseudomonas syringae strains. Systemic acquired resistance and systemic PATHOGENESIS-RELATED GENE1 induction were also compromised in cds2-1D. The T-DNA insertion adjacent to NINE-CIS-EPOXYCAROTENOID DIOXYGENASE5 (NCED5), one of six genes encoding the abscisic acid (ABA) biosynthetic enzyme NCED, caused a massive increase in transcript level and enhanced ABA levels >2-fold. Overexpression of NCED genes recreated the enhanced disease susceptibility phenotype. NCED2, NCED3, and NCED5 were induced, and ABA accumulated strongly following compatible P. syringae infection. The ABA biosynthetic mutant aba3-1 showed reduced susceptibility to virulent P. syringae, and ABA, whether through exogenous application or endogenous accumulation in response to mild water stress, resulted in increased bacterial growth following challenge with virulent P. syringae, indicating that ABA suppresses resistance to P. syringae. Likewise ABA accumulation also compromised resistance to the biotrophic oomycete Hyaloperonospora arabidopsis, whereas resistance to the fungus Alternaria brassicicola was enhanced in cds2-1D plants and compromised in aba3-1 plants, indicating that ABA promotes resistance to this necrotroph. Comparison of the accumulation of salicylic acid and jasmonic acid in the wild type, cds2-1D, and aba3-1 plants challenged with P. syringae showed that ABA promotes jasmonic acid accumulation and exhibits a complex antagonistic relationship with salicylic acid. Our findings provide genetic evidence that the abiotic stress signal ABA also has profound roles in modulating diverse plant-pathogen interactions mediated at least in part by cross talk with the jasmonic acid and salicylic acid biotic stress signal pathways.
Figures

References
-
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460–3479 - PMC - PubMed
-
- Asselbergh B, De Vleesschauwer D, Hofte M (2008. a) Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21 709–719 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

