Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors
- PMID: 19571576
- PMCID: PMC3073484
- DOI: 10.1159/000226224
Effects of pathological flow on pulmonary artery endothelial production of vasoactive mediators and growth factors
Abstract
Background: Alterations in pulmonary blood flow are often associated with the initiation and progression of pulmonary vascular disease. However, the cellular mechanisms involved in mediating flow effects in the pulmonary circulation remain unclear. Depending on the disease condition, flow may be extremely low or high. We therefore examined effects of pathologically low and high flow on endothelial production of factors capable of affecting pulmonary vascular tone and structure as well as on potential underlying mechanisms.
Methods: Flow effects on pulmonary endothelial release of NO, PGF(1a), ET-1 and TxB(2), on expression of total and phosphorylated eNOS as well as Akt, and on VEGF were examined. Additionally, in a coculture system, effects of flow-exposed endothelial cells on smooth muscle (SM) proliferation and contractile protein were studied.
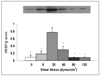
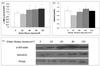
Results: Compared to physiological flow, pathologically high and low flow attenuated endothelial release of NO and PGF(1a), and enhanced release of ET-1. Physiological flow activated the Akt/eNOS pathway, while pathological flow depressed it. Pathologically high flow altered VE-cadherin expression. Pathologically high flow on the endothelium upregulated alpha-SM-actin and SM-MHC without affecting SM proliferation.
Conclusion: Physiological flow leads to production of mediators which favor vasodilation. Pathological flow alters the balance of mediator production which favors vasoconstriction.
Copyright 2009 S. Karger AG, Basel.
Figures



References
-
- Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2005;39:492–503. - PubMed
-
- Cool CD, Groshong SD, Oakey J, Voelkel NF. Pulmonary hypertension: cellular and molecular mechanisms. Chest. 2005;128:565S–571S. - PubMed
-
- Botney MD. Role of hemodynamics in pulmonary vascular remodeling: implications for primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:361–364. - PubMed
-
- Ben Driss A, Devaux C, Henrion D, Duriez M, Thuillez C, Levy BI, Michel JB. Hemodynamic stresses induce endothelial dysfunction and remodeling of pulmonary artery in experimental compensated heart failure. Circulation. 2000;101:2764–2770. - PubMed
-
- Storme L, Parker TA, Kinsella JP, Rairigh RL, Abman SH. Chronic hypertension impairs flow-induced vasodilation and augments the myogenic response in fetal lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L56–L66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

