Prophylaxis and therapy for Chikungunya virus infection
- PMID: 19572805
- PMCID: PMC7109959
- DOI: 10.1086/600381
Prophylaxis and therapy for Chikungunya virus infection
Abstract
Background: Chikungunya virus (CHIKV) is a recently reemerged arbovirus responsible for a massive outbreak of infection in the Indian Ocean region and India that has a very significant potential to spread globally because of the worldwide distribution of its mosquito vectors. CHIKV induces a usually self-limited disease in humans that is characterized by fever, arthralgia, myalgia, and rash; however, cases of severe CHIKV infection have recently been described, particularly in adults with underlying condition and neonates born to viremic mothers.
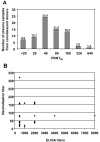
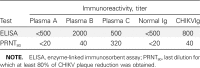
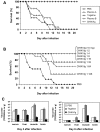
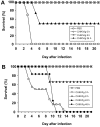
Methods: Human polyvalent immunoglobulins were purified from plasma samples obtained from donors in the convalescent phase of CHIKV infection, and the preventive and curative effects of these immunoglobulins were investigated in 2 mouse models of CHIKV infection that we developed.
Results: CHIKV immunoglobulins contain anti-CHIKV antibodies and exhibit a high in vitro neutralizing activity and a powerful prophylactic and therapeutic efficacy against CHIKV infection in vivo, including in the neonate.
Conclusions: Administration of CHIKV immunoglobulins may constitute a safe and efficacious prevention strategy and treatment for individuals exposed to CHIKV who are at risk of severe infection, such as neonates born to viremic mothers and adults with underlying conditions. These results provide a proof-of-concept for purifying human immunoglobulins from plasma samples from patients in the convalescent phase of an emerging infectious disease for which neither prevention nor treatment is available.
Figures

Comment in
-
Chikungunya: first steps toward specific treatment and prophylaxis.J Infect Dis. 2009 Aug 15;200(4):489-91. doi: 10.1086/600382. J Infect Dis. 2009. PMID: 19572806 No abstract available.
References
-
- Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg. 49:28–32. - PubMed
-
- Enserink M. Infectious diseases: massive outbreak draws fresh attention to little-known virus. Science. 311:1085. - PubMed
-
- Bonn D. How did Chikungunya reach the Indian Ocean? Lancet Infect Dis. 6:543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

