Increased radioresistance and accelerated B cell lymphomas in mice with Mdmx mutations that prevent modifications by DNA-damage-activated kinases
- PMID: 19573810
- PMCID: PMC2758524
- DOI: 10.1016/j.ccr.2009.05.008
Increased radioresistance and accelerated B cell lymphomas in mice with Mdmx mutations that prevent modifications by DNA-damage-activated kinases
Abstract
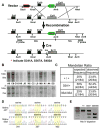
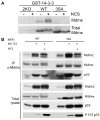
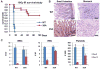
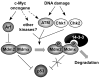
Mdmx is a critical negative regulator of the p53 pathway that is stoichiometrically limiting in some tissues. Posttranslational modification and degradation of Mdmx after DNA damage have been proposed to be essential for p53 activation. We tested this model in vivo, where critical stoichiometric relationships are preserved. We generated an Mdmx mutant mouse in which three conserved serines (S341, S367, S402) targeted by DNA-damage-activated kinases were replaced by alanines to investigate whether modifications of these residues are important for Mdmx degradation and p53 activation. The mutant mice were remarkably resistant to radiation, and very susceptible to Myc-induced lymphomagenesis. These data demonstrate that Mdmx downregulation is crucial for effective p53-mediated radiation responses and tumor suppression in vivo.
Figures



Comment in
-
Tumour suppression: Full on.Nat Rev Cancer. 2009 Sep;9(9):612. doi: 10.1038/nrc2725. Nat Rev Cancer. 2009. PMID: 19705529 No abstract available.
References
-
- Adams J, Harris A, Pinkert C, Corcoran L, Alexander W, Cory S, Palmiter R, Brinster R. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318(6046):533–8. - PubMed
-
- Alitalo K, Koskinen P, Mäkelä TP, Saksela K, Sistonen L, Winqvist R. myc oncogenes: activation and amplification. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1987;907(1):1. - PubMed
-
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864. - PubMed
-
- Burdelya LG, Komarova EA, Hill JE, Browder T, Tararova ND, Mavrakis L, DiCorleto PE, Folkman J, Gudkov AV. Inhibition of p53 Response in Tumor Stroma Improves Efficacy of Anticancer Treatment by Increasing Antiangiogenic Effects of Chemotherapy and Radiotherapy in Mice. Cancer Res. 2006;66(19):9356–9361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

