Evidence for catalytic roles for Plasmodium falciparum aminopeptidase P in the food vacuole and cytosol
- PMID: 19574214
- PMCID: PMC2757184
- DOI: 10.1074/jbc.M109.018424
Evidence for catalytic roles for Plasmodium falciparum aminopeptidase P in the food vacuole and cytosol
Abstract
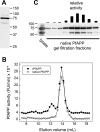
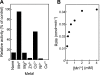
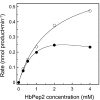
The metalloenzyme aminopeptidase P catalyzes the hydrolysis of amino acids from the amino termini of peptides with a prolyl residue in the second position. The human malaria parasite Plasmodium falciparum expresses a homolog of aminopeptidase P during its asexual intraerythrocytic cycle. P. falciparum aminopeptidase P (PfAPP) shares with mammalian cytosolic aminopeptidase P a three-domain, homodimeric organization and is most active with Mn(II) as the cofactor. A distinguishing feature of PfAPP is a 120-amino acid amino-terminal extension that appears to be removed from the mature protein. PfAPP is present in the food vacuole and cytosol of the parasite, a distribution that suggests roles in vacuolar hemoglobin catabolism and cytosolic peptide turnover. To evaluate the plausibility of these putative functions, the stability and kinetic properties of recombinant PfAPP were evaluated at the acidic pH of the food vacuole and at the near-neutral pH of the cytosol. PfAPP exhibited high stability at 37 degrees C in the pH range 5.0-7.5. In contrast, recombinant human cytosolic APP1 was unstable and formed a high molecular weight aggregate at acidic pH. At both acidic and slightly basic pH values, PfAPP efficiently hydrolyzed the amino-terminal X-Pro bond of the nonapeptide bradykinin and of two globin pentapeptides that are potential in vivo substrates. These results provide support for roles for PfAPP in peptide catabolism in both the food vacuole and the cytosol and suggest that PfAPP has evolved a dual distribution in response to the metabolic needs of the intraerythrocytic parasite.
Figures


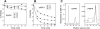
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

