The functional curli amyloid is not based on in-register parallel beta-sheet structure
- PMID: 19574225
- PMCID: PMC2757210
- DOI: 10.1074/jbc.M109.007054
The functional curli amyloid is not based on in-register parallel beta-sheet structure
Abstract
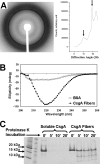
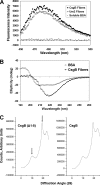
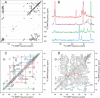
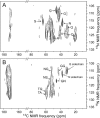
The extracellular curli proteins of Enterobacteriaceae form fibrous structures that are involved in biofilm formation and adhesion to host cells. These curli fibrils are considered a functional amyloid because they are not a consequence of misfolding, but they have many of the properties of protein amyloid. We confirm that fibrils formed by CsgA and CsgB, the primary curli proteins of Escherichia coli, possess many of the hallmarks typical of amyloid. Moreover we demonstrate that curli fibrils possess the cross-beta structure that distinguishes protein amyloid. However, solid state NMR experiments indicate that curli structure is not based on an in-register parallel beta-sheet architecture, which is common to many human disease-associated amyloids and the yeast prion amyloids. Solid state NMR and electron microscopy data are consistent with a beta-helix-like structure but are not sufficient to establish such a structure definitively.
Figures







References
-
- Virchow R. (1853) C. R. Acad. Sci. 37, 860–861
-
- Stefani M., Dobson C. M. (2003) J. Mol. Med. 81, 678–699 - PubMed
-
- Fowler D. M., Koulov A. V., Balch W. E., Kelly J. W. (2007) Trends Biochem. Sci. 32, 217–224 - PubMed
-
- Podrabsky J. E., Carpenter J. F., Hand S. C. (2001) Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R123–R131 - PubMed
-
- Mackay J. P., Matthews J. M., Winefield R. D., Mackay L. G., Haverkamp R. G., Templeton M. D. (2001) Structure 9, 83–91 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

