Proteolytic processing of HCN2 and co-assembly with HCN4 in the generation of cardiac pacemaker channels
- PMID: 19574228
- PMCID: PMC2757956
- DOI: 10.1074/jbc.M109.007583
Proteolytic processing of HCN2 and co-assembly with HCN4 in the generation of cardiac pacemaker channels
Abstract
In sino-atrial and atrio-ventricular nodal cells, hyperpolarization-activated cyclic nucleotide-gated (HCN) inward current carrying cationic channels, I(f), are expressed that contribute importantly to the diastolic depolarization critical for cardiac pacemaker activity. Although previous studies have demonstrated myocardial expression of both the HCN2 and HCN4 subunits, the specific roles of these subunits in the generation of functional myocardial I(f) channels remain unclear. To explore the molecular compositions of functional cardiac I(f) channels, antibodies targeted against specific C- and N-terminal sequences in HCN2 and HCN4 were exploited to examine HCN2 and HCN4 subunit expression in adult (mouse) heart and to immunoprecipitate endogenous HCN-encoded cardiac I(f) channel complexes. Western blot experiments revealed that although the full-length HCN2 (105 kDa) and HCN4 (160 kDa) proteins are readily detected in transiently transfected HEK-293 cells and in adult (mouse) brain, the molecular mass of the HCN2 protein in the myocardium is approximately 60 kDa. In addition, the myocardial 60-kDa HCN2 protein lacks the C terminus, which contains the cAMP binding domain. In heterologous cells, the C-terminal-truncated HCN2 protein co-assembles with HCN4 to form functional heteromeric HCN channels, which activate faster than homomeric HCN2 or homomeric HCN4 channels, and display properties similar to endogenous myocardial I(f) channels Taken together, these results suggest that functional myocardial I(f) channels reflect the heteromeric assembly of HCN2 and HCN4 subunits and further that the HCN4 subunit underlies the cAMP-mediated regulation of cardiac I(f) channels.
Figures



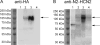

Comment in
-
Non-proteolytic HCN2 in the heart.J Biol Chem. 2009 Sep 25;284(39):le7; author reply le8. doi: 10.1074/jbc.L109.007583. J Biol Chem. 2009. PMID: 19767400 Free PMC article. No abstract available.
Similar articles
-
Associated changes in HCN2 and HCN4 transcripts and I(f) pacemaker current in myocytes.Biochim Biophys Acta. 2009 May;1788(5):1138-47. doi: 10.1016/j.bbamem.2009.02.011. Epub 2009 Feb 21. Biochim Biophys Acta. 2009. PMID: 19236845 Free PMC article.
-
Single-channel properties support a potential contribution of hyperpolarization-activated cyclic nucleotide-gated channels and If to cardiac arrhythmias.Circulation. 2005 Feb 1;111(4):399-404. doi: 10.1161/01.CIR.0000153799.65783.3A. Circulation. 2005. PMID: 15687126
-
Transcriptional control of pacemaker channel genes HCN2 and HCN4 by Sp1 and implications in re-expression of these genes in hypertrophied myocytes.Cell Physiol Biochem. 2009;23(4-6):317-26. doi: 10.1159/000218178. Epub 2009 May 6. Cell Physiol Biochem. 2009. PMID: 19471099
-
HCN-related channelopathies.Pflugers Arch. 2010 Jul;460(2):405-15. doi: 10.1007/s00424-010-0810-8. Epub 2010 Mar 8. Pflugers Arch. 2010. PMID: 20213494 Review.
-
Pacemaker channels and sinus node arrhythmia.Trends Cardiovasc Med. 2004 Jan;14(1):23-8. doi: 10.1016/j.tcm.2003.09.006. Trends Cardiovasc Med. 2004. PMID: 14720471 Review.
Cited by
-
The fast and slow ups and downs of HCN channel regulation.Channels (Austin). 2010 May-Jun;4(3):215-31. doi: 10.4161/chan.4.3.11630. Channels (Austin). 2010. PMID: 20305382 Free PMC article. Review.
-
Cytoplasmic Autoinhibition in HCN Channels is Regulated by the Transmembrane Region.J Membr Biol. 2020 Apr;253(2):153-166. doi: 10.1007/s00232-020-00111-8. Epub 2020 Mar 7. J Membr Biol. 2020. PMID: 32146488 Free PMC article.
-
HCN Channel C-Terminal Region Speeds Activation Rates Independently of Autoinhibition.J Membr Biol. 2015 Dec;248(6):1043-60. doi: 10.1007/s00232-015-9816-7. Epub 2015 Jun 30. J Membr Biol. 2015. PMID: 26123597
-
The integration and functional evaluation of rabbit pacing cells transplanted into the left ventricular free wall.Int J Med Sci. 2012;9(7):513-20. doi: 10.7150/ijms.4971. Epub 2012 Aug 27. Int J Med Sci. 2012. PMID: 22991489 Free PMC article.
-
The brain is required for normal muscle and nerve patterning during early Xenopus development.Nat Commun. 2017 Sep 25;8(1):587. doi: 10.1038/s41467-017-00597-2. Nat Commun. 2017. PMID: 28943634 Free PMC article.
References
-
- DiFrancesco D. (1993) Annu. Rev. Physiol. 55, 455–472 - PubMed
-
- Herrmann S., Stieber J., Ludwig A. (2007) Pflugers Arch. 454, 517–522 - PubMed
-
- Barbuti A., DiFrancesco D. (2008) Ann. N.Y. Acad. Sci. 1123, 213–223 - PubMed
-
- Pape H. C. (1996) Annu. Rev. Physiol. 58, 299–327 - PubMed
-
- Santoro B., Liu D. T., Yao H., Bartsch D., Kandel E. R., Siegelbaum S. A., Tibbs G. R. (1998) Cell 93, 717–729 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

