Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease
- PMID: 19574425
- PMCID: PMC2716955
- DOI: 10.2353/ajpath.2009.081011
Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease
Abstract
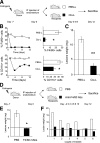
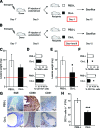
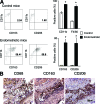
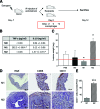
The mechanisms that sustain endometrial tissues at ectopic sites in patients with endometriosis are poorly understood. Various leukocytes, including macrophages, infiltrate endometriotic lesions. In this study, we depleted mouse macrophages by means of either clodronate liposomes or monoclonal antibodies before the injection of syngeneic endometrial tissue. In the absence of macrophages, tissue fragments adhered and implanted into the peritoneal wall, but endometriotic lesions failed to organize and develop. When we depleted macrophages after the establishment of endometriotic lesions, blood vessels failed to reach the inner layers of the lesions, which stopped growing. Macrophages from patients with endometriosis and experimental mice, but not nonendometriotic patients who underwent surgery for uterine leiomyomas or control mice, expressed markers of alternative activation. These markers included high levels of scavenger receptors, CD163 and CD206, which are involved in both the scavenging of hemoglobin with iron transfer into macrophages and the silent clearance of inflammatory molecules. Macrophages in both inflammatory liquid and ectopic lesions were equally polarized, suggesting a critical role of environmental cues in the peritoneal cavity. Adoptively transferred, alternatively activated macrophages dramatically enhanced endometriotic lesion growth in mice. Inflammatory macrophages effectively protected mice from endometriosis. Therefore, endogenous macrophages involved in tissue remodeling appear as players in the natural history of endometriosis, required for effective vascularization and ectopic lesion growth.
Figures


References
-
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. - PubMed
-
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. - PubMed
-
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–1589. - PubMed
-
- Sampson J. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469.
-
- Dunselman GA, Groothuis PG. Etiology of endometriosis: hypotheses and facts. Gynecol Obstet Invest. 2004;57:42–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

