Development of arterial blood supply in experimental liver metastases
- PMID: 19574433
- PMCID: PMC2716978
- DOI: 10.2353/ajpath.2009.090095
Development of arterial blood supply in experimental liver metastases
Abstract
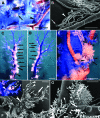
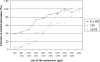
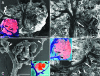
In this study, we present a mechanism for the development of arterial blood supply in experimental liver metastases. To analyze the arterialization process of experimental liver metastases, we elucidated a few key questions regarding the blood supply of hepatic lobules in mice. The microvasculature of the mouse liver is characterized by numerous arterioportal anastomoses and arterial terminations at the base of the lobules. These terminations supply one hepatic microcirculatory subunit per lobule, which we call an arterial hepatic microcirculatory subunit (aHMS). The process of arterialization can be divided into the following steps: 1) distortion of the aHMS by metastasis; 2) initial fusion of the sinusoids of the aHMS at the tumor parenchyma interface; 3) fusion of the sinusoids located at the base of the aHMSs, which leads to the disruption of the vascular sphincter (burst pipe); 4) incorporation of the dilated artery and the fused sinusoids into the tumor; and 5) further development of the tumor vasculature (arterial tree) by proliferation, remodeling, and continuous incorporation of fused sinusoids at the tumor-parenchyma interface. This process leads to the inevitable arterialization of liver metastases above the 2000- to 2500-mum size, regardless of the origin and growth pattern of the tumor.
Figures





References
-
- Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surg. 1989;76:545–548. - PubMed
-
- Lin G, Lunderquist A, Hägerstrand I, Boijsen E. Postmortem examination of the blood supply and vascular pattern of small liver metastases in man. Surgery. 1984;96:517–526. - PubMed
-
- Ackerman NB. The blood supply of experimental liver metastases. IV Changes in vascularity with increasing tumor growth. Surgery. 1974;75:589–596. - PubMed
-
- Ridge JA, Bading JR, Gelbard AS, Benua RS, Daly JM. Perfusion of colorectal hepatic metastases. Relative distribution of flow from the hepatic artery and portal vein. Cancer. 1987;59:1547–1553. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

