Deletion of Pten expands lung epithelial progenitor pools and confers resistance to airway injury
- PMID: 19574443
- PMCID: PMC2778148
- DOI: 10.1164/rccm.200901-0100OC
Deletion of Pten expands lung epithelial progenitor pools and confers resistance to airway injury
Abstract
Rationale: Pten is a tumor-suppressor gene involved in stem cell homeostasis and tumorigenesis. In mouse, Pten expression is ubiquitous and begins as early as 7 days of gestation. Pten(-/-) mouse embryos die early during gestation indicating a critical role for Pten in embryonic development.
Objectives: To test the role of Pten in lung development and injury.
Methods: We conditionally deleted Pten throughout the lung epithelium by crossing Pten(flox/flox) with Nkx2.1-cre driver mice. The resulting Pten(Nkx2.1-cre) mutants were analyzed for lung defects and response to injury.
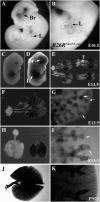
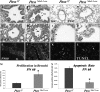
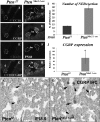
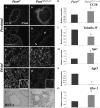
Measurements and main results: Pten(Nkx2.1-cre) embryonic lungs showed airway epithelial hyperplasia with no branching abnormalities. In adult mice, Pten(Nkx2.1-cre) lungs exhibit increased progenitor cell pools composed of basal cells in the trachea, CGRP/CC10 double-positive neuroendocrine cells in the bronchi, and CC10/SPC double-positive cells at the bronchioalveolar duct junctions. Pten deletion affected differentiation of various lung epithelial cell lineages, with a decreased number of terminally differentiated cells. Over time, Pten(Nxk2.1-cre) epithelial cells residing in the bronchioalveolar duct junctions underwent proliferation and formed uniform masses, supporting the concept that the cells residing in this distal niche may also be the source of procarcinogenic stem cells. Finally, increased progenitor cells in all the lung compartments conferred an overall selective advantage to naphthalene injury compared with wild-type control mice.
Conclusions: Pten has a pivotal role in lung stem cell homeostasis, cell differentiation, and consequently resistance to lung injury.
Figures






Similar articles
-
Embryonic epithelial Pten deletion through Nkx2.1-cre leads to thyroid tumorigenesis in a strain-dependent manner.Endocr Relat Cancer. 2012 Apr 10;19(2):111-122. doi: 10.1530/ERC-10-0327. Print 2012 Apr. Endocr Relat Cancer. 2012. PMID: 22167068 Free PMC article.
-
Region-specific role for Pten in maintenance of epithelial phenotype and integrity.Am J Physiol Lung Cell Mol Physiol. 2017 Jan 1;312(1):L131-L142. doi: 10.1152/ajplung.00005.2015. Epub 2016 Nov 18. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27864284 Free PMC article.
-
Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice.J Clin Invest. 2007 Oct;117(10):2929-40. doi: 10.1172/JCI31854. J Clin Invest. 2007. PMID: 17909629 Free PMC article.
-
Stem/progenitor cells in lung development, injury repair, and regeneration.Proc Am Thorac Soc. 2008 Aug 15;5(6):703-6. doi: 10.1513/pats.200801-012AW. Proc Am Thorac Soc. 2008. PMID: 18684721 Free PMC article. Review.
-
Portrait of PTEN: messages from mutant mice.Cancer Sci. 2008 Feb;99(2):209-13. doi: 10.1111/j.1349-7006.2007.00670.x. Epub 2008 Jan 15. Cancer Sci. 2008. PMID: 18201277 Free PMC article. Review.
Cited by
-
Epithelial Wntless regulates postnatal alveologenesis.Development. 2022 Jan 1;149(1):dev199505. doi: 10.1242/dev.199505. Epub 2022 Jan 10. Development. 2022. PMID: 34931663 Free PMC article.
-
PTEN: An Emerging Potential Target for Therapeutic Intervention in Respiratory Diseases.Oxid Med Cell Longev. 2022 Jun 30;2022:4512503. doi: 10.1155/2022/4512503. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35814272 Free PMC article. Review.
-
Cross-talk between transforming growth factor-beta and Wingless/Int pathways in lung development and disease.Int J Biochem Cell Biol. 2010 Jun;42(6):809-12. doi: 10.1016/j.biocel.2010.02.011. Epub 2010 Feb 26. Int J Biochem Cell Biol. 2010. PMID: 20219694 Free PMC article. Review.
-
Embryonic epithelial Pten deletion through Nkx2.1-cre leads to thyroid tumorigenesis in a strain-dependent manner.Endocr Relat Cancer. 2012 Apr 10;19(2):111-122. doi: 10.1530/ERC-10-0327. Print 2012 Apr. Endocr Relat Cancer. 2012. PMID: 22167068 Free PMC article.
-
Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia.Am J Respir Cell Mol Biol. 2014 Feb;50(2):253-62. doi: 10.1165/rcmb.2013-0114OC. Am J Respir Cell Mol Biol. 2014. PMID: 24010952 Free PMC article.
References
-
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004;116:769–778. - PubMed
-
- Lanza R. Essentials of stem cells biology. New York: Academic Press; 2006.
-
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995;121:2031–2046. - PubMed
-
- Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC, Randell SH. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol 2004;286:L631–L642. - PubMed
-
- Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E, Péault B. Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells 2005;23:992–1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

