Cyclin D1 promotes anchorage-independent cell survival by inhibiting FOXO-mediated anoikis
- PMID: 19575018
- PMCID: PMC3071150
- DOI: 10.1038/cdd.2009.86
Cyclin D1 promotes anchorage-independent cell survival by inhibiting FOXO-mediated anoikis
Erratum in
- Cell Death Differ. 2010 May;17(5):900
Abstract
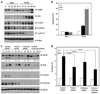
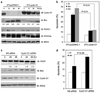
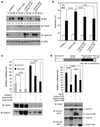
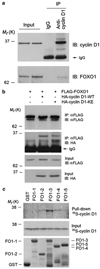
O-class forkhead box (FOXO) transcription factors are critical regulators of diverse cellular processes, including apoptosis, cell-cycle arrest, DNA damage repair and oxidative stress resistance. Here, we show that FOXO1 and FOXO3a have an essential function in promoting cell detachment-induced anoikis, resistance to which is implicated in cancer development and metastasis. In contrast, the oncoprotein cyclin D1 inhibits anoikis. We further show that cyclin D1 interacts with FOXO proteins and impedes their transcriptional regulatory and anoikis-promoting functions. This effect of cyclin D1 requires its transcription repression domain but is independent of cyclin-dependent kinases CDK4 and CDK6. Moreover, we show that cancer-derived mutants of cyclin D1 are much more stable than wild-type cyclin D1 under anchorage-independent conditions and possess a greater antagonistic effect on FOXO-regulated anoikis and anchorage-independent growth of cancer cells. These data suggest that cyclin D1 may have a critical function in tumorigenesis and cancer metastasis by inhibiting the anoikis-promoting function of FOXO proteins.
Conflict of interest statement
All authors claim no conflict of interest with this study.
Figures
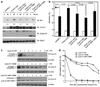
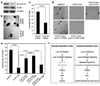
References
-
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. - PubMed
-
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. - PubMed
-
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

