Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production
- PMID: 19575365
- PMCID: PMC2722691
- DOI: 10.1002/hep.23019
Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production
Abstract
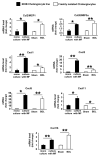
The mechanisms mediating hepatic accumulation of inflammatory cells in cholestatic liver disease remain enigmatic. Our thesis is that Hedgehog (Hh) pathway activation promotes hepatic accumulation of immune cells that interact with cholangiocytes. We believe that myofibroblastic hepatic stellate cells (MF-HSCs) release soluble Hh ligands that stimulate cholangiocytes to express chemokines that recruit mononuclear cell types with cognate receptors for these chemokines, thereby orchestrating a repair-related mechanism for liver inflammation. To address this thesis, we used three experimental systems that allow the definition of Hh-dependent mechanisms that induce phenotypic changes in cholangiocytes. First, cholangiocytes were cultured alone or in the presence of Hh-producing MF-HSCs in a transwell coculture system and/or treated with MF-HSC-conditioned medium with or without Hh-neutralizing antibodies. Changes in the cholangiocyte phenotype were then evaluated by microarray analysis, quantitative reverse-transcriptase polymerase chain reaction (QRT-PCR), and/or enzyme-linked immunosorbent assay for chemokine (C-X-C) motif ligand 16 (Cxcl16). Bile duct ligation was chosen to model biliary fibrosis in mice with an overly active Hh pathway, control littermates, and healthy rats, and the gene profile was evaluated by QRT-PCR in whole liver tissue. Second, a transwell chemotaxis assay was used to examine natural killer T (NKT) cell migration in response to cholangiocytes and particularly cholangiocyte-derived Cxcl16. Finally, we studied liver samples from primary biliary cirrhosis patients and controls by QRT-PCR to compare differences in the Hh pathway and Cxcl16. Co-immunostaining of cytokeratin-7 and Cxcl16 was then performed to localize the phenotypic source of Cxcl16. We found that MF-HSCs release soluble Hh ligands that stimulate cholangiocytes to produce Cxcl16 and recruit NKT cells. Hh pathway activation during cholestatic liver injury also induces cholangiocyte expression of Cxcl16.
Conclusion: During biliary injury, Hh pathway activation induces cholangiocyte production of chemokines that recruit NKT cells to portal tracts.
Figures




References
-
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. - PubMed
-
- Marra F. Chemokines in liver inflammation and fibrosis. Front Biosci. 2002;7:d1899–1914. - PubMed
-
- Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond) 2007;112:265–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
