Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase
- PMID: 19575460
- PMCID: PMC2914599
- DOI: 10.1002/hep.23059
Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase
Abstract
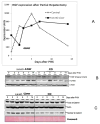
Following liver regeneration after partial hepatectomy, liver grows back precisely to its original mass and does not exceed it. The mechanism regulating this "hepatostat" is not clear and no exceptions have been found to date. Although pathways initiating liver regeneration have been well studied, mechanisms involved in the termination of liver regeneration are unclear. Here, we report that integrin-linked kinase (ILK) (involved in transmission of the extracellular matrix [ECM] signaling by way of integrin receptors) and/or hepatic adaptations that ensue following ILK hepatocyte-targeted removal are critical for proper termination of liver regeneration. Following partial hepatectomy (PHx), mice with a liver-specific ILK ablation (ILK-KO-Liver) demonstrate a termination defect resulting in 58% larger liver than their original pre-PHx mass. This increase in post-PHx liver mass is due to sustained cell proliferation driven in part by increased signaling through hepatocyte growth factor (HGF), and the beta-catenin pathway and Hippo kinase pathways.
Conclusion: The data indicate that ECM-mediated signaling by way of ILK is essential in proper termination of liver regeneration. This is the first evidence of a defect leading to impaired termination of regeneration and excessive accumulation of liver weight following partial hepatectomy.
Figures




References
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. - PubMed
-
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. - PubMed
-
- Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, et al. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857–867. - PubMed
-
- Oe S, Lemmer ER, Conner EA, Factor VM, Leveen P, Larsson J, Karlsson S, et al. Intact signaling by transforming growth factor beta is not required for termination of liver regeneration in mice. Hepatology. 2004;40:1098–1105. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
