Membrane traffic within the Golgi apparatus
- PMID: 19575639
- PMCID: PMC2877624
- DOI: 10.1146/annurev.cellbio.24.110707.175421
Membrane traffic within the Golgi apparatus
Abstract
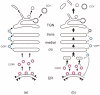
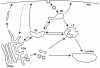
Newly synthesized secretory cargo molecules pass through the Golgi apparatus while resident Golgi proteins remain in the organelle. However, the pathways of membrane traffic within the Golgi are still uncertain. Most of the available data can be accommodated by the cisternal maturation model, which postulates that Golgi cisternae form de novo, carry secretory cargoes forward and ultimately disappear. The entry face of the Golgi receives material that has been exported from transitional endoplasmic reticulum sites, and the exit face of the Golgi is intimately connected with endocytic compartments. These conserved features are enhanced by cell-type-specific elaborations such as tubular connections between mammalian Golgi cisternae. Key mechanistic questions remain about the formation and maturation of Golgi cisternae, the recycling of resident Golgi proteins, the origins of Golgi compartmental identity, the establishment of Golgi architecture, and the roles of Golgi structural elements in membrane traffic.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

