Rolling cell adhesion
- PMID: 19575676
- PMCID: PMC3557855
- DOI: 10.1146/annurev.cellbio.042308.113238
Rolling cell adhesion
Abstract
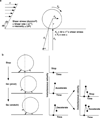
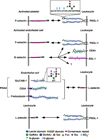
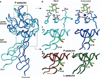
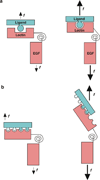
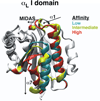
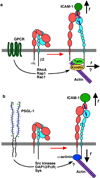
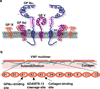
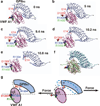
Rolling adhesion on vascular surfaces is the first step in recruiting circulating leukocytes, hematopoietic progenitors, or platelets to specific organs or to sites of infection or injury. Rolling requires the rapid yet balanced formation and dissociation of adhesive bonds in the challenging environment of blood flow. This review explores how structurally distinct adhesion receptors interact through mechanically regulated kinetics with their ligands to meet these challenges. Remarkably, increasing force applied to adhesive bonds first prolongs their lifetimes (catch bonds) and then shortens their lifetimes (slip bonds). Catch bonds mediate the counterintuitive phenomenon of flow-enhanced rolling adhesion. Force-regulated disruptions of receptor interdomain or intradomain interactions remote from the ligand-binding surface generate catch bonds. Adhesion receptor dimerization, clustering in membrane domains, and interactions with the cytoskeleton modulate the forces applied to bonds. Both inside-out and outside-in cell signals regulate these processes.
Figures








References
-
- Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin: carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995a;374:539–542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL090923/HL/NHLBI NIH HHS/United States
- AI077343/AI/NIAID NIH HHS/United States
- HL085607/HL/NHLBI NIH HHS/United States
- HL093723/HL/NHLBI NIH HHS/United States
- R01 HL091020/HL/NHLBI NIH HHS/United States
- R21 AI044902/AI/NIAID NIH HHS/United States
- HL091020/HL/NHLBI NIH HHS/United States
- AI44902/AI/NIAID NIH HHS/United States
- R01 AI044902/AI/NIAID NIH HHS/United States
- HL34363/HL/NHLBI NIH HHS/United States
- R37 HL034363/HL/NHLBI NIH HHS/United States
- R01 HL090923/HL/NHLBI NIH HHS/United States
- P01 HL085607/HL/NHLBI NIH HHS/United States
- R01 HL093723/HL/NHLBI NIH HHS/United States
- R01 HL034363/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

