Vertebrate endoderm development and organ formation
- PMID: 19575677
- PMCID: PMC2861293
- DOI: 10.1146/annurev.cellbio.042308.113344
Vertebrate endoderm development and organ formation
Abstract
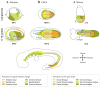
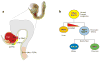
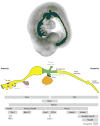
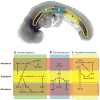
The endoderm germ layer contributes to the respiratory and gastrointestinal tracts and to all of their associated organs. Over the past decade, studies in vertebrate model organisms, including frog, fish, chick, and mouse, have greatly enhanced our understanding of the molecular basis of endoderm organ development. We review this progress with a focus on early stages of endoderm organogenesis including endoderm formation, gut tube morphogenesis and patterning, and organ specification. Lastly, we discuss how developmental mechanisms that regulate endoderm organogenesis are used to direct differentiation of embryonic stem cells into specific adult cell types, which function to alleviate disease symptoms in animal models.
Figures





References
-
- Alexander J, Stainier DY. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–57. - PubMed
-
- Aoki TO, David NB, Minchiotti G, Saint-Etienne L, Dickmeis T, et al. Molecular integration of casanova in the Nodal signaling pathway controlling endoderm formation. Development. 2002;129:275–86. - PubMed
-
- Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

