A novel method for subarachnoid hemorrhage to induce vasospasm in mice
- PMID: 19576247
- PMCID: PMC2769994
- DOI: 10.1016/j.jneumeth.2009.06.027
A novel method for subarachnoid hemorrhage to induce vasospasm in mice
Abstract
Mouse models take advantage of genetic manipulations that can be achieved in this species. There are currently two accepted mouse models of subarachnoid hemorrhage (SAH) and cerebral vasospasm (CVs). Both are technically demanding and labor intensive. In this study, we report a reproducible and technically feasible method to induce SAH, and subsequently CVs, in mice. We tested this model in multiple strains of mice that are commonly used for genetic manipulation.
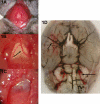
Methods: SAH was induced in C57BL/6NCr, FVB, 129S1, BalbC and SJL mice, weighing 28-32 g, by an intracisternal vessel transection technique. Animals were perfused with India ink at 24h postprocedure and vessel diameters were quantified. Brain slices were obtained for hematoxylin-eosin staining (H&E) to look for vascular changes consistent with CVs.
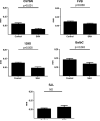
Results: There was no mortality during or after the procedure. Four of the five mouse strains showed significant CVs at 24 h postprocedure characterized by decreased vessel diameter of the middle cerebral artery close to the Circle of Willis. Histologically, the vessel wall displayed significant corrugation and thickening, consistent with CVs.
Conclusion: A novel mouse model to induce SAH is described and tested in several mouse strains. Four of the five strains used in this study developed CVs after the induction of SAH. The procedure is brief, straightforward, reproducible with low mortality, and applicable to commonly used background strains for genetically engineered mice.
Figures

References
-
- Acalovschi D, Wiest T, Hartmann M, Farahmi M, Mansmann U, Auffarth GU, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–9. - PubMed
-
- Bagley C. Blood in the cerebrospinal fluid: resultant functional and organic alterations in the central nervous system. Part A. Experimental data. Arch Surg. 1928;17:18–38.
-
- Hochmeister S, Grundtner R, Bauer J, Engelhardt B, Lyck R, Gordon G, et al. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65:855–65. - PubMed
-
- Kamii H, Kato I, Kinouchi H, Chan PH, Epstein CJ, Akabane A, et al. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn-superoxide dismutase. Stroke. 1999;30:867–71. [discussion 72] - PubMed
-
- Lin C L, Calisaneller T, Ukita N, Dumont AS, Kassell NF, Lee KS. A murine model of subarachnoid hemorrhage-induced cerebral vasospasm. J Neurosci Methods. 2003;123:89–97. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

