Hematopoietic stem cell origin of adipocytes
- PMID: 19576951
- PMCID: PMC2740899
- DOI: 10.1016/j.exphem.2009.06.008
Hematopoietic stem cell origin of adipocytes
Abstract
Objective: It has generally been believed that adipocytes are derived from mesenchymal stem cells via fibroblasts. We recently reported that fibroblasts/myofibroblasts in a number of tissues and organs are derived from hematopoietic stem cells (HSCs). In the present study, we tested the hypothesis that HSCs also give rise to adipocytes.
Materials and methods: Using transplantation of a single enhanced green fluorescent protein-positive (EGFP(+)) HSC and primary culture, we examined generation of adipocytes from HSCs.
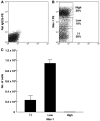
Results: Adipose tissues from clonally engrafted mice showed EGFP(+) adipocytes that stained positive for leptin, perilipin, and fatty acid binding protein 4. A diet containing rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, significantly enhanced the number of EGFP(+) adipocytes. When EGFP(+) bone marrow cells from clonally engrafted mice were cultured under adipogenic conditions, all of the cultured cells stained positive with Oil Red O and Sudan Black B and exhibited the presence of abundant mRNA for adipocyte markers. Finally, clonal culture- and sorting-based studies of Mac-1 expression of hematopoietic progenitors suggested that adipocytes are derived from HSCs via progenitors for monocytes/macrophages.
Conclusion: Together, these studies clarify the current controversy regarding the ability of HSCs to give rise to adipocytes. Furthermore, our primary culture method that generates adipocytes from uncommitted hematopoietic cells should contribute to the studies of the mechanisms of early adipocytic differentiation and may lead to development of therapeutic solutions for many general obesity issues.
Conflict of interest statement
Figures







References
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
-
- Gregoire FM. Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. - PubMed
-
- Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–3126S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

