Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier
- PMID: 19578006
- PMCID: PMC2736779
- DOI: 10.1681/ASN.2008121286
Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier
Abstract
Podocytes possess the complete machinery for glutamatergic signaling, raising the possibility that neuron-like signaling contributes to glomerular function. To test this, we studied mice and cells lacking Rab3A, a small GTPase that regulates glutamate exocytosis. In addition, we blocked the glutamate ionotropic N-methyl-d-aspartate receptor (NMDAR) with specific antagonists. In mice, the absence of Rab3A and blockade of NMDAR both associated with an increased urinary albumin/creatinine ratio. In humans, NMDAR blockade, obtained by addition of ketamine to general anesthesia, also had an albuminuric effect. In vitro, Rab3A-null podocytes displayed a dysregulated release of glutamate with higher rates of spontaneous exocytosis, explained by a reduction in Rab3A effectors resulting in freedom of vesicles from the actin cytoskeleton. In addition, NMDAR antagonism led to profound cytoskeletal remodeling and redistribution of nephrin in cultured podocytes; the addition of the agonist NMDA reversed these changes. In summary, these results suggest that glutamatergic signaling driven by podocytes contributes to the integrity of the glomerular filtration barrier and that derangements in this signaling may lead to proteinuric renal diseases.
Figures
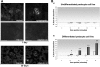
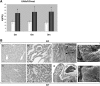
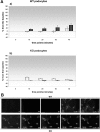
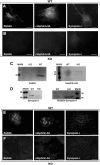
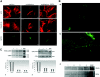
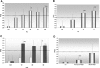
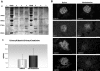
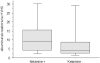
Comment in
-
Signaling at the slit: podocytes chat by synaptic transmission.J Am Soc Nephrol. 2009 Sep;20(9):1862-4. doi: 10.1681/ASN.2009070691. Epub 2009 Jul 23. J Am Soc Nephrol. 2009. PMID: 19628665 No abstract available.
References
-
- Tryggvason K, Patrakka J, Wartiovaara J:Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 - PubMed
-
- Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K:The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 - PubMed
-
- Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR:Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

