Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study
- PMID: 19578087
- PMCID: PMC2714688
- DOI: 10.1136/bmj.b2480
Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study
Abstract
Context: Whether immunosuppressive treatment adversely affects survival is unclear.
Objective: To assess whether immunosuppressive drugs increase mortality.
Design: Retrospective cohort study evaluating overall and cancer mortality in relation to immunosuppressive drug exposure among patients with ocular inflammatory diseases. Demographic, clinical, and treatment data derived from medical records, and mortality results from United States National Death Index linkage. The cohort's mortality risk was compared with US vital statistics using standardised mortality ratios. Overall and cancer mortality in relation to use or non-use of immunosuppressive drugs within the cohort was studied with survival analysis.
Setting: Five tertiary ocular inflammation clinics. Patients 7957 US residents with non-infectious ocular inflammation, 2340 of whom received immunosuppressive drugs during follow up. Exposures Use of antimetabolites, T cell inhibitors, alkylating agents, and tumour necrosis factor inhibitors.
Main outcome measures: Overall mortality, cancer mortality.
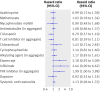
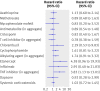
Results: Over 66 802 person years (17 316 after exposure to immunosuppressive drugs), 936 patients died (1.4/100 person years), 230 (24.6%) from cancer. For patients unexposed to immunosuppressive treatment, risks of death overall (standardised mortality ratio 1.02, 95% confidence interval [CI] 0.94 to 1.11) and from cancer (1.10, 0.93 to 1.29) were similar to those of the US population. Patients who used azathioprine, methotrexate, mycophenolate mofetil, ciclosporin, systemic corticosteroids, or dapsone had overall and cancer mortality similar to that of patients who never took immunosuppressive drugs. In patients who used cyclophosphamide, overall mortality was not increased and cancer mortality was non-significantly increased. Tumour necrosis factor inhibitors were associated with increased overall (adjusted hazard ratio [HR] 1.99, 95% CI 1.00 to 3.98) and cancer mortality (adjusted HR 3.83, 1.13 to 13.01).
Conclusions: Most commonly used immunosuppressive drugs do not seem to increase overall or cancer mortality. Our results suggesting that tumour necrosis factor inhibitors might increase mortality are less robust than the other findings; additional evidence is needed.
Conflict of interest statement
Competing interests: JTR has received ≤$10 000 for consulting fees or paid advisory boards for Abbott Laboratories (ciclosporin, adalimumab) and Novartis (ciclosporin), and has a research grant from Abbott Laboratories; EBS has received a research grant from Abbott Laboratories; DAJ has received ≤$10 000 for consulting fees or paid advisory boards for Novartis. JTR and EBS have received a research grant from Centocor (infliximab); CSF reports having received fees of ≤$10 000 for speaking at the invitation of Centocor. JTR reports equity ownership/stock options >$10 000 in Amgen (etanercept). JHK, JPD, CSF, JTR, and EBS previously participated in a clinical trial of a competing product, fluocinolone acetonide implant, sponsored by Bausch & Lomb; JHK, JPD, CSF, DAJ, RBN, and JET are participating in an NIH-sponsored study comparing this product with systemic therapy for uveitis, and GAL-C previously participated in this trial; Bausch & Lomb is providing a limited amount of drug product in support of this study.
Figures
Comment in
-
Imuunosuppressants, mortality, and risk of cancer.BMJ. 2009 Jul 3;339:b1645. doi: 10.1136/bmj.b1645. BMJ. 2009. PMID: 19578084 No abstract available.
References
-
- Van den Borne BE, Landewe RB, Houkes I, Schild F, van der Heyden PC, Hazes JM, et al. No increased risk of malignancies and mortality in cyclosporin A-treated patients with rheumatoid arthritis. Arthritis Rheum 1998;41:1930-7. - PubMed
-
- Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481-94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources