Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease
- PMID: 19578116
- PMCID: PMC2749124
- DOI: 10.1074/jbc.M109.012393
Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease
Abstract
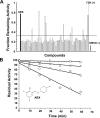
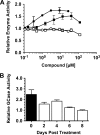
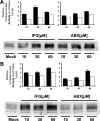
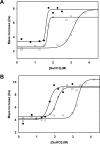
Gaucher disease (GD), the most prevalent lysosomal storage disease, is caused by a deficiency of glucocerebrosidase (GCase). The identification of small molecules acting as agents for enzyme enhancement therapy is an attractive approach for treating different forms of GD. A thermal denaturation assay utilizing wild type GCase was developed to screen a library of 1,040 Food and Drug Administration-approved drugs. Ambroxol (ABX), a drug used to treat airway mucus hypersecretion and hyaline membrane disease in newborns, was identified and found to be a pH-dependent, mixed-type inhibitor of GCase. Its inhibitory activity was maximal at neutral pH, found in the endoplasmic reticulum, and undetectable at the acidic pH of lysosomes. The pH dependence of ABX to bind and stabilize the enzyme was confirmed by monitoring the rate of hydrogen/deuterium exchange at increasing guanidine hydrochloride concentrations. ABX treatment significantly increased N370S and F213I mutant GCase activity and protein levels in GD fibroblasts. These increases were primarily confined to the lysosome-enriched fraction of treated cells, a finding confirmed by confocal immunofluorescence microscopy. Additionally, enhancement of GCase activity and a reduction in glucosylceramide storage was verified in ABX-treated GD lymphoblasts (N370S/N370S). Hydrogen/deuterium exchange mass spectrometry revealed that upon binding of ABX, amino acid segments 243-249, 310-312, and 386-400 near the active site of GCase are stabilized. Consistent with its mixed-type inhibition of GCase, modeling studies indicated that ABX interacts with both active and non-active site residues. Thus, ABX has the biochemical characteristics of a safe and effective enzyme enhancement therapy agent for the treatment of patients with the most common GD genotypes.
Figures




References
-
- Sidransky E. (1997) Adv. Pediatr. 44,73–107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

