Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression
- PMID: 19578162
- PMCID: PMC2789773
- DOI: 10.1194/jlr.M900255-JLR200
Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression
Abstract
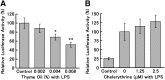
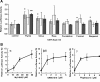
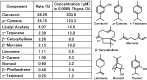
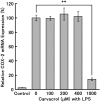
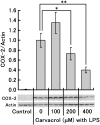
Cyclooxygenase-2 (COX-2), the rate-limiting enzyme in prostaglandin biosynthesis, plays a key role in inflammation and circulatory homeostasis. Peroxisome proliferator-activated receptors (PPARs) are ligand-dependent transcription factors belonging to the nuclear receptor superfamily and are involved in the control of COX-2 expression, and vice versa. Here, we show that COX-2 promoter activity was suppressed by essential oils derived from thyme, clove, rose, eucalyptus, fennel, and bergamot in cell-based transfection assays using bovine arterial endothelial cells. Moreover, from thyme oil, we identified carvacrol as a major component of the suppressor of COX-2 expression and an activator of PPARalpha and gamma. PPARgamma-dependent suppression of COX-2 promoter activity was observed in response to carvacrol treatment. In human macrophage-like U937 cells, carvacrol suppressed lipopolysaccharide-induced COX-2 mRNA and protein expression, suggesting that carvacrol regulates COX-2 expression through its agonistic effect on PPARgamma. These results may be important in understanding the antiinflammatory and antilifestyle-related disease properties of carvacrol.
Figures





References
-
- Smith W. L. 2008. Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem. Sci. 33: 27–33. - PubMed
-
- Simmons D. L., Botting R. M., Hla T. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56: 387–437. - PubMed
-
- Koki A., Khan N. K., Woerner B. M., Dannenberg A. J., Olson L., Seibert K., Edwards D., Hardy M., Isakson P., Masferrer J. L. 2002. Cyclooxygenase-2 in human pathological disease. Adv. Exp. Med. Biol. 507: 177–184. - PubMed
-
- Dubois R. N., Abramson S. B., Crofford L., Gupta R. A., Simon L. S., Van De Putte L. B., Lipsky P. E. 1998. Cyclooxygenase in biology and disease. FASEB J. 12: 1063–1073. - PubMed
-
- Morham S. G., Langenbach R., Loftin C. D., Tiano H. F., Vouloumanos N., Jennette J. C., Mahler J. F., Kluckman K. D., Ledford A., Lee C. A., et al. 1995. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 83: 473–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

