Mass spectrometry of membrane transporters reveals subunit stoichiometry and interactions
- PMID: 19578383
- PMCID: PMC4066579
- DOI: 10.1038/nmeth.1347
Mass spectrometry of membrane transporters reveals subunit stoichiometry and interactions
Abstract
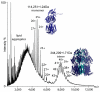
We describe a general mass spectrometry approach to determine subunit stoichiometry and lipid binding in intact membrane protein complexes. By exploring conditions for preserving interactions during transmission into the gas phase and for optimally stripping away detergent, by subjecting the complex to multiple collisions, we released the intact complex largely devoid of detergent. This enabled us to characterize both subunit stoichiometry and lipid binding in 4 membrane protein complexes.
Figures


References
-
- Schagger H, von Jagow G. Anal. Biochem. 1991;199:223–231. - PubMed
-
- Burgess NK, Stanley AM, Fleming KG. Methods Cell Biol. 2008;84:181–211. - PubMed
-
- Putnam CD, Hammel M, Hura GL, Tainer JA. Q. Rev. Biophys. 2007;40:191–285. - PubMed
-
- Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Science. 2008;321:243–246. - PubMed
-
- Locher KP, Lee AT, Rees DC. Science. 2002;296:1091–1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

