Effects of Cyclooxygenase Inhibitors on Apoptotic Neuroretinal Cells
- PMID: 19578520
- PMCID: PMC2688370
- DOI: 10.4137/bmi.s692
Effects of Cyclooxygenase Inhibitors on Apoptotic Neuroretinal Cells
Abstract
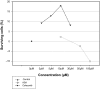
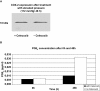
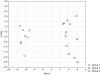
Glaucoma is characterized by a loss of retinal ganglion cells (RGC) which is associated with a decrease of visual function. Neuroprotective agents as a new therapeutic strategy could prevent the remaining neurons from apoptotic cell death. Previous studies have shown the involvement of the Cyclooxygenase (COX)-2 signalling in the apoptotic death of neurons. Herein we investigated the neuroprotective effect of COX-1/COX-2- and selective COX-2- inhibitors on apoptotic. R28, a neuroretinal cell line and determined the PGE(2) levels by ELISA. Furthermore we investigated differences in protein expression in the cells after exposure to elevated pressure compared to untreated cells by ProteinChip analysis.In addition, a protein profiling study of the cells after exposure to elevated pressure was performed. The protein expression profiles were measured by SELDI-TOF (Surface Enhanced Laser Desorption/Ionization-time of flight) Protein Chips. The protein identification was performed by mass spectrometry (MS).It could be shown that COX-2 inhibition significantly prevented the cells from apoptosis and reduced the PGE(2) concentrations. Selective COX-2 inhibitors were significant more potent than non-selective inhibitors or COX-1 inhibitors. We found differently expressed protein patterns in neuroretinal cells cultured at atmospheric pressure compared to those cells exposed to elevated pressure with or without celecoxib respectively. We identified three biomarkers, ubiquitin, HSP10 and NDKB, which were differently expressed in the groups. However, our data indicates a distinct neuroprotective effect of COX-2 inhibition. The local treatment with selective COX-2 inhibitors might provide an innovative strategy of therapeutic intervention for glaucoma.
Figures







Similar articles
-
Protective effect of 14-3-3 antibodies on stressed neuroretinal cells via the mitochondrial apoptosis pathway.BMC Ophthalmol. 2015 Jun 27;15:64. doi: 10.1186/s12886-015-0044-9. BMC Ophthalmol. 2015. PMID: 26115916 Free PMC article.
-
The role of COX-2/PGE2 in gossypol-induced apoptosis of colorectal carcinoma cells.J Cell Physiol. 2012 Aug;227(8):3128-37. doi: 10.1002/jcp.23067. J Cell Physiol. 2012. PMID: 22170686
-
Cyclooxygenase-2, player or spectator in cyclooxygenase-2 inhibitor-induced apoptosis in prostate cancer cells.J Natl Cancer Inst. 2002 Apr 17;94(8):585-91. doi: 10.1093/jnci/94.8.585. J Natl Cancer Inst. 2002. PMID: 11959891
-
Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis.Prog Exp Tumor Res. 2003;37:179-92. doi: 10.1159/000071373. Prog Exp Tumor Res. 2003. PMID: 12795055 Review.
-
Surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI TOF-MS) and ProteinChip technology in proteomics research.Pathol Res Pract. 2004;200(2):83-94. doi: 10.1016/j.prp.2004.01.010. Pathol Res Pract. 2004. PMID: 15237917 Review.
Cited by
-
Oroxylin A promotes retinal ganglion cell survival in a rat optic nerve crush model.PLoS One. 2017 Jun 22;12(6):e0178584. doi: 10.1371/journal.pone.0178584. eCollection 2017. PLoS One. 2017. PMID: 28640893 Free PMC article.
-
Protective Effects of Human Pericyte-like Adipose-Derived Mesenchymal Stem Cells on Human Retinal Endothelial Cells in an In Vitro Model of Diabetic Retinopathy: Evidence for Autologous Cell Therapy.Int J Mol Sci. 2023 Jan 4;24(2):913. doi: 10.3390/ijms24020913. Int J Mol Sci. 2023. PMID: 36674425 Free PMC article.
-
The Potential Mechanism of Eriodictyol in Treating Alzheimer's Disease: A Study on Computer-assisted Investigational Strategies.Curr Pharm Des. 2024;30(26):2086-2107. doi: 10.2174/0113816128304628240526071425. Curr Pharm Des. 2024. PMID: 38920073
-
The large conductance calcium-activated potassium channel affects extrinsic and intrinsic mechanisms of apoptosis.J Neurosci Res. 2015 May;93(5):745-54. doi: 10.1002/jnr.23538. Epub 2015 Jan 7. J Neurosci Res. 2015. PMID: 25581503 Free PMC article.
-
Protective effect of resveratrol on retinal damage in glaucoma: a systematic review and meta-analysis of preclinical studies.Front Pharmacol. 2025 Jan 15;15:1521188. doi: 10.3389/fphar.2024.1521188. eCollection 2024. Front Pharmacol. 2025. PMID: 39881870 Free PMC article.
References
-
- Agar A, Shaojuan L, Agarwal N, Coroneo MT, Hill MA. Retinal ganglion cell line apoptosis induced by hydrostatic pressure. . Brain Res. 2006;1086:191–200. - PubMed
-
- Bagetta G, Corasaniti MT, Paoletti AM, Berliocchi L, Nistico R, Giammarioli AM, Malorni W, Finazzi-Agro A. HIV-1 gp 120-induced apoptosis in the rat neocortex involves enhanced expression of cyclooxygenase type 2 (COX-2). . Biochem. Biophys. Res. Commun. 1998;244:819–24. - PubMed
-
- Bazan NG. The neuromessenger platelet-activating factor in plasticity and neurodegeneration. . Prog. Brain Res. 1998;118:281–91. - PubMed
-
- Bazan NG. COX-2 as a multifunctional neuronal modulator. . Nat. Med. 2001;7:414–5. - PubMed
-
- Cazevieille C, Muller A, Meynier F, Dutrait N, Bonne C. Protection by prostaglandins from glutamate toxicity in cortical neurons. . Neurochem. Int. 1994;24:395–408. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

