A serum- and feeder-free technique of culturing human corneal epithelial stem cells on amniotic membrane
- PMID: 19578552
- PMCID: PMC2704912
A serum- and feeder-free technique of culturing human corneal epithelial stem cells on amniotic membrane
Abstract
Purpose: To describe a simple technique of cultivating human corneal epithelial stem cells using an Epilife culture medium under serum- and feeder-free conditions.
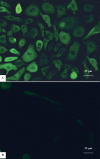
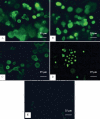
Methods: Cadaveric donor limbal corneal epithelial cells were cultured on denuded amniotic membranes using an explant technique that was free of serum and feeder cells in the Epilife medium containing a growth supplement of defined composition. These cells were assessed by phase contrast microscope. The expressions of the proposed corneal epithelial stem cell markers (p63, ATP-binding cassette member 2 (ABCG2), and cytokeratin 15 and 19) and differentiation markers (cytokeratin 3, 12, connexin 43, and p75) were analyzed using reverse transcription polymerase chain reaction (RT-PCR) and immunocytochemical staining.
Results: Successful cultures were obtained, resulting in a monolayer to double layer cell sheets with a cobblestone-like morphology. RT-PCR and immunocytochemistry disclosed an expression of both putative limbal stem cell (LSC) markers and differentiation-associated markers in the cultured cells. Most of the cultured corneal epithelial cells that were immunopositive for putative LSC markers were smaller, more uniform, and closer to the limbal explant than cells positively stained with differentiation-associated markers.
Conclusions: A serum- and feeder-free culture system using Epilife medium may grow human corneal epithelial equivalents, minimizing the risk of contamination during culture. The technique may also be useful for the clinical application of limbal stem cell culture.
Figures



References
-
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. - PubMed
-
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–3. - PubMed
-
- Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985;26:1296–9. - PubMed
-
- Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
