Optimal velocity and safety of discontinuous conduction through the heterogeneous Purkinje-ventricular junction
- PMID: 19580741
- PMCID: PMC2711367
- DOI: 10.1016/j.bpj.2009.03.061
Optimal velocity and safety of discontinuous conduction through the heterogeneous Purkinje-ventricular junction
Abstract
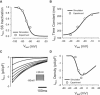
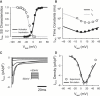
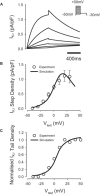
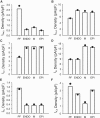
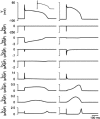
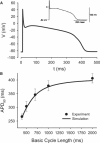
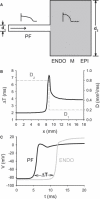
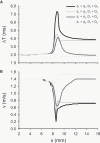
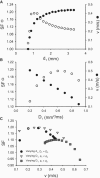
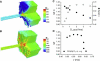
Slow and discontinuous wave conduction through nonuniform junctions in cardiac tissues is generally considered unsafe and proarrythmogenic. However, the relationships between tissue structure, wave conduction velocity, and safety at such junctions are unknown. We have developed a structurally and electrophysiologically detailed model of the canine Purkinje-ventricular junction (PVJ) and varied its heterogeneity parameters to determine such relationships. We show that neither very fast nor very slow conduction is safe, and there exists an optimal velocity that provides the maximum safety factor for conduction through the junction. The resultant conduction time delay across the PVJ is a natural consequence of the electrophysiological and morphological differences between the Purkinje fiber and ventricular tissue. The delay allows the PVJ to accumulate and pass sufficient charge to excite the adjacent ventricular tissue, but is not long enough for the source-to-load mismatch at the junction to be enhanced over time. The observed relationships between the conduction velocity and safety factor can provide new insights into optimal conditions for wave propagation through nonuniform junctions between various cardiac tissues.
Figures




Comment in
-
Cardiac muscle is not a uniform syncytium.Biophys J. 2010 Jun 16;98(12):3102-3; discussion 3104-5. doi: 10.1016/j.bpj.2010.03.030. Biophys J. 2010. PMID: 20550924 Free PMC article. No abstract available.
References
-
- Tyson J.J., Keener J.P. Singular perturbation theory of traveling waves in excitable media (a review) Physica D. 1988;32:327–361.
-
- Zipes D.P., Jalife J., editors. Cardiac Electrophysiology. From Cell to Bedside Sanders; Philadelphia: 2004.
-
- Shaw R.M., Rudy Y. Ionic mechanisms of propagation in cardiac tissue: roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ. Res. 1997;81:727–741. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

