Influence of the charge at D85 on the initial steps in the photocycle of bacteriorhodopsin
- PMID: 19580764
- PMCID: PMC2711356
- DOI: 10.1016/j.bpj.2009.04.021
Influence of the charge at D85 on the initial steps in the photocycle of bacteriorhodopsin
Abstract
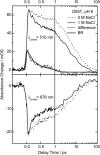
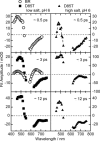
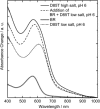
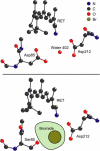
Studies have shown that trans-cis isomerization of retinal is the primary photoreaction in the photocycle of the light-driven proton pump bacteriorhodopsin (BR) from Halobacterium salinarum, as well as in the photocycle of the chloride pump halorhodopsin (HR). The transmembrane proteins HR and BR show extensive structural similarities, but differ in the electrostatic surroundings of the retinal chromophore near the protonated Schiff base. Point mutation of BR of the negatively charged aspartate D85 to a threonine T (D85T) in combination with variation of the pH value and anion concentration is used to study the ultrafast photoisomerization of BR and HR for well-defined electrostatic surroundings of the retinal chromophore. Variations of the pH value and salt concentration allow a switch in the isomerization dynamics of the BR mutant D85T between BR-like and HR-like behaviors. At low salt concentrations or a high pH value (pH 8), the mutant D85T shows a biexponential initial reaction similar to that of HR. The combination of high salt concentration and a low pH value (pH 6) leads to a subpopulation of 25% of the mutant D85T whose stationary and dynamic absorption properties are similar to those of native BR. In this sample, the combination of low pH and high salt concentration reestablishes the electrostatic surroundings originally present in native BR, but only a minor fraction of the D85T molecules have the charge located exactly at the position required for the BR-like fast isomerization reaction. The results suggest that the electrostatics in the native BR protein is optimized by evolution. The accurate location of the fixed charge at the aspartate D85 near the Schiff base in BR is essential for the high efficiency of the primary reaction.
Figures




References
-
- Haupts U., Tittor J., Oesterhelt D. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct. 1999;28:367–399. - PubMed
-
- Lanyi J.K., Duschl A., Hatfield G.W., May K., Oesterhelt D. Understanding structure and function in the light-driven proton pump bacteriorhodopsin. J. Struct. Biol. 1998;124:164–178. - PubMed
-
- Lanyi J.K. Bacteriorhodopsin. Annu. Rev. Physiol. 2004;66:665–688. - PubMed
-
- Oesterhelt D. Structure and function of halorhodopsin. Isr. J. Chem. 1995;35:475–494.
-
- Lanyi J.K. Halorhodopsin: a light-driven chloride ion pump. Annu. Rev. Biophys. Biophys. Chem. 1986;15:11–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

