Modulation of calcium oxalate dihydrate growth by selective crystal-face binding of phosphorylated osteopontin and polyaspartate peptide showing occlusion by sectoral (compositional) zoning
- PMID: 19581305
- PMCID: PMC2749123
- DOI: 10.1074/jbc.M109.021899
Modulation of calcium oxalate dihydrate growth by selective crystal-face binding of phosphorylated osteopontin and polyaspartate peptide showing occlusion by sectoral (compositional) zoning
Abstract
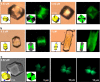
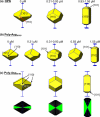
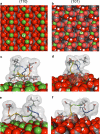
Calcium oxalate dihydrate (COD) mineral and the urinary protein osteopontin/uropontin (OPN) are commonly found in kidney stones. To investigate the effects of OPN on COD growth, COD crystals were grown with phosphorylated OPN or a polyaspartic acid-rich peptide of OPN (DDLDDDDD, poly-Asp(86-93)). Crystals grown with OPN showed increased dimensions of the {110} prismatic faces attributable to selective inhibition at this crystallographic face. At high concentrations of OPN, elongated crystals with dominant {110} faces were produced, often with intergrown, interpenetrating twin crystals. Poly-Asp(86-93) dose-dependently elongated crystal morphology along the {110} faces in a manner similar to OPN. In crystal growth studies using fluorescently tagged poly-Asp(86-93) followed by imaging of crystal interiors using confocal microscopy, sectoral (compositional) zoning in COD was observed resulting from selective binding and incorporation (occlusion) of peptide exclusively into {110} crystal sectors. Computational modeling of poly-Asp(86-93) adsorption to COD {110} and {101} surfaces also suggests increased stabilization of the COD {110} surface and negligible change to the natively stable {101} surface. Ultrastructural, colloidal-gold immunolocalization of OPN by transmission electron microscopy in human stones confirmed an intracrystalline distribution of OPN. In summary, OPN and its poly-Asp(86-93) sequence similarly affect COD mineral growth; the {110} crystallographic faces become enhanced and dominant attributable to {110} face inhibition by the protein/peptide, and peptides can incorporate into the mineral phase. We, thus, conclude that the poly-Asp(86-93) domain is central to the OPN ability to interact with the {110} faces of COD, where it binds to inhibit crystal growth with subsequent intracrystalline incorporation (occlusion).
Figures



Similar articles
-
The effect of intracrystalline and surface-bound osteopontin on the degradation and dissolution of calcium oxalate dihydrate crystals in MDCKII cells.Urol Res. 2012 Feb;40(1):1-15. doi: 10.1007/s00240-011-0423-5. Epub 2011 Sep 20. Urol Res. 2012. PMID: 21932131
-
Modulation of calcium oxalate dihydrate growth by phosphorylated osteopontin peptides.J Struct Biol. 2018 Nov;204(2):131-144. doi: 10.1016/j.jsb.2018.07.010. Epub 2018 Jul 17. J Struct Biol. 2018. PMID: 30016645
-
The effect of intracrystalline and surface-bound osteopontin on the attachment of calcium oxalate dihydrate crystals to Madin-Darby canine kidney (MDCK) cells in ultrafiltered human urine.BJU Int. 2012 Apr;109(7):1100-9. doi: 10.1111/j.1464-410X.2011.10530.x. Epub 2011 Aug 24. BJU Int. 2012. PMID: 21883862
-
Molecular modulation of calcium oxalate crystallization.Am J Physiol Renal Physiol. 2006 Dec;291(6):F1123-31. doi: 10.1152/ajprenal.00136.2006. Am J Physiol Renal Physiol. 2006. PMID: 17082348 Review.
-
Role of crystal surface adhesion in kidney stone disease.Curr Opin Nephrol Hypertens. 2006 Jul;15(4):386-93. doi: 10.1097/01.mnh.0000232879.50716.6f. Curr Opin Nephrol Hypertens. 2006. PMID: 16775453 Review.
Cited by
-
Intrinsically Disordered Osteopontin Fragment Orders During Interfacial Calcium Oxalate Mineralization.Angew Chem Int Ed Engl. 2021 Aug 16;60(34):18577-18581. doi: 10.1002/anie.202105768. Epub 2021 Jul 16. Angew Chem Int Ed Engl. 2021. PMID: 34118104 Free PMC article.
-
The effect of intracrystalline and surface-bound osteopontin on the degradation and dissolution of calcium oxalate dihydrate crystals in MDCKII cells.Urol Res. 2012 Feb;40(1):1-15. doi: 10.1007/s00240-011-0423-5. Epub 2011 Sep 20. Urol Res. 2012. PMID: 21932131
-
3D visualization of additive occlusion and tunable full-spectrum fluorescence in calcite.Nat Commun. 2016 Nov 18;7:13524. doi: 10.1038/ncomms13524. Nat Commun. 2016. PMID: 27857076 Free PMC article.
-
Biomolecular mechanism of urinary stone formation involving osteopontin.Urol Res. 2012 Dec;40(6):623-37. doi: 10.1007/s00240-012-0514-y. Epub 2012 Nov 6. Urol Res. 2012. PMID: 23124115 Review.
-
Relative deficiency of acidic isoforms of osteopontin from stone former urine.Urol Res. 2012 Oct;40(5):447-54. doi: 10.1007/s00240-012-0459-1. Epub 2012 Feb 10. Urol Res. 2012. PMID: 22322528 Free PMC article.
References
-
- Wesson J. A., Worcester E. M., Wiessner J. H., Mandel N. S., Kleinman J. G. (1998) Kidney Int. 53,952–957 - PubMed
-
- Mandel N. S., Mandel G. S. (1989) J. Urol. 142,1516–1521 - PubMed
-
- Durrbaum D., Rodgers A. L., Sturrock E. D. (2001) Urol. Res. 29,83–88 - PubMed
-
- Mandel N. S., Mandel G. S., Hasegawa A. T. (1987) J. Urol. 138,557–562 - PubMed
-
- Cerini C., Geider S., Dussol B., Hennequin C., Daudon M., Veesler S., Nitsche S., Boistelle R., Berthézène P., Dupuy P., Vazi A., Berland Y., Dagorn J. C., Verdier J. M. (1999) Kidney Int. 55,1776–1786 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

