Gr1+ cells control growth of YopM-negative yersinia pestis during systemic plague
- PMID: 19581396
- PMCID: PMC2738001
- DOI: 10.1128/IAI.00284-09
Gr1+ cells control growth of YopM-negative yersinia pestis during systemic plague
Abstract
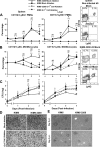
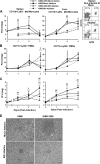
YopM, a protein toxin of Yersinia pestis, is necessary for virulence in a mouse model of systemic plague. We previously reported YopM-dependent natural killer (NK) cell depletion from blood and spleen samples of infected mice. However, in this study we found that infection with Y. pestis KIM5 (YopM(+)) caused depletion of NK cells in the spleen, but not in the liver, and antibody-mediated ablation of NK cells had no effect on bacterial growth. There was no YopM-associated effect on the percentage of dendritic cells (DCs) or polymorphonuclear leukocytes (PMNs) in the early stage of infection; however, there was a YopM-associated effect on PMN integrity and on the influx of monocytes into the spleen. Ablation of Gr1(+) cells caused loss of the growth defect of YopM(-) Y. pestis in both the liver and spleen. In contrast, ablation of macrophages/DCs inhibited growth of both parent and mutant bacteria, accompanied by significantly fewer lesion sites in the liver. These results point toward PMNs and inflammatory monocytes as major cell types that control growth of YopM(-) Y. pestis. Infection with fully virulent Y. pestis CO92 and a YopM(-) derivative by intradermal and intranasal routes showed that the absence of YopM significantly increased the 50% lethal dose only in the intradermal model, suggesting a role for YopM in bubonic plague, in which acute inflammation occurs soon after infection.
Figures




References
-
- Achtman, M., G. Morelli, P. Zhu, T. Wirth, I. Diehl, B. Kusecek, A. J. Vogler, D. M. Wagner, C. J. Allender, W. R. Easterday, V. Chenal-Francisque, P. Worsham, N. R. Thomson, J. Parkhill, L. E. Lindler, E. Carniel, and P. Keim. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc. Natl. Acad. Sci. USA 10117837-17842. - PMC - PubMed
-
- Ardavin, C. 2003. Origin, precursors and differentiation of mouse dendritic cells. Nat. Rev. Immunol. 3582-590. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. - PubMed
-
- Bendelac, A., P. B. Savage, and L. Teyton. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25297-336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

